Lecture 5
advertisement

Announcements
1) Revised Lab timings: 1-3 PM (all groups)
2) Quiz 1, 28th Jan 2014,
Tuesday 7:30 PM, WS 209, WS 213
Chapter 3
Crystal Geometry
and
Structure Determination
Recap
Lattice, Motif/Basis
Crystal = Lattice + Motif
e.g. Brass, diamond, ZnS
Miller indices of direction: components of vector w.r.t
to basis vector a, b and c
Miller Indices of directions and
planes
William Hallowes Miller
(1801 – 1880)
University of Cambridge
z
Miller Indices for planes
1. Select a crystallographic
coordinate system with origin not
on the plane
2. Find intercepts along
axes in terms of respective
lattice parameters
O
x
y
1 1 1
3. Take reciprocal 1 1 1
4. Convert to smallest integers in
the same ratio
1 1 1
5. Enclose in parenthesis
(111)
Miller Indices for planes (contd.)
z
z
E
Plane
ABCD
OCBE
origin
O
O*
intercepts 1
∞ ∞
reciprocals 1 0 0
A
B
O
O*
y
D
x
C
x
Miller
Indices
(1 0 0)
Zero
represents that
the plane is
parallel to the
corresponding
axis
1 -1
∞
1 -1 0
_
(1 1 0)
Bar
represents
a negative
intercept
Courtesy: H Bhadhesia
Courtesy: H Bhadhesia
Courtesy: H Bhadhesia
Crystallographically equivalent planes
Miller indices of a family of symmetry related planes
{hkl }
= (hkl ) and all other planes related to
(hkl ) by the symmetry of the crystal
All the faces of the cube
are equivalent to each
other by symmetry
Front & back faces: (100)
Left and right faces: (010)
Top and bottom faces: (001)
{100} = (100), (010), (001)
Miller indices of a family of
symmetry related planes
Cubic
z
Tetragonal
z
y
y
x
{100}cubic = (100), (010), (001)
x
{100}tetragonal = (100), (010)
(001)
CUBIC CRYSTALS
[111]
[hkl] (hkl)
(111)
Angle between two directions [h1k1l1] and [h2k2l2]:
cos
h1h2 k1k2 l1l2
h12 k12 l12 h22 k22 l22
C
Some IMPORTANT Results
Weiss zone law
Not in the textbook
• If a direction [uvw] lies in a plane (hkl) then
• uh+vk+wl = 0
(hkl)
True for ALL crystal systems
dhkl
Interplanar spacing
between ‘successive’ (hkl)
planes passing through the
corners of the unit cell
cubic
d hkl
a
h 2 k 2 l 2
z
E
B
O
O
d100 a
x
(100)
d1 1 0
x
a
2
Summary of Notation convention for
Indices
[uvw]
Miller indices of a direction (i.e. a set
of parallel directions)
(hkl)
Miller Indices of a plane (i.e. a set of
parallel planes)
<uvw>
Miller indices of a family of symmetry
related directions
{hkl}
Miller indices of a family of symmetry
related planes
How do we determine the
structure of a piece of crystalline
solid?
You can probe the atomic
arrangements by X-ray diffraction
(XRD)
X-Ray Diffraction
≡ Bragg Reflection
Sample
Incident Beam
Transmitted
Beam
Braggs Law (Part 1): For every diffracted
beam there exists a set of crystal lattice
planes such that the diffracted beam
appears to be specularly reflected from this
set of planes.
X-Ray Diffraction
Braggs Law (Part 1): the diffracted beam
appears to be specularly reflected from a set of
crystal lattice planes.
Specular reflection:
Angle of incidence
i
=Angle of reflection
(both measured from the
plane
plane and not from
the normal)
r
The incident beam, the reflected beam and the
plane normal lie in one plane
X-Ray Diffraction
r
i
dhkl
Bragg’s law (Part 2):
n 2dhkl sin
r
i
P
R
dhkl
Q
Path Difference =PQ+QR 2d hkl sin
i
r
P
R
Q
Path Difference =PQ+QR 2d hkl sin
Constructive inteference
n 2dhkl sin
Bragg’s law
Extinction Rules: Table 3.3
Bravais Lattice
Allowed Reflections
SC
All
BCC
(h + k + l) even
FCC
h, k and l unmixed
h, k and l are all odd
DC
Or
if all are even then
(h + k + l) divisible by 4
Diffraction analysis of cubic crystals
Bragg’s Law:
2d hkl sin
(1)
Cubic crystals
d hkl
(2)
a
h2 k 2 l 2
2
sin
(2) in (1) =>
2
4a 2
(h 2 k 2 l 2 )
constant
2
2
2
sin
(h k l )
2
X Ray Diffractometer
You do not get indices of plane!!
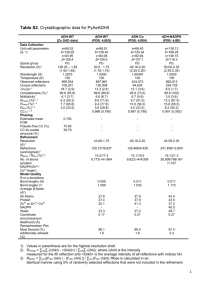
Cu target, Wavelength = 1.5418 Angstrom
2θ
44.48
Unknown sample, cubic
51.83
76.35
92.90
98.40
121.87
144.54
Determine:
1) The crystal structure
2) Lattice parameter
5 step program for the determination of crystal structure
1) Start with 2θ values and generate a set of sin2θ values
2) Normalise the sin2θ values by dividing it with first entry
3) Clear fractions from normalised column: Multiply by
common number
4) Speculate on the hkl values that, if expressed as
h2+k2+l2, would generate the sequence of the “clear
fractions” column
5) Compute for each sin2θ /(h2+k2+l2) on the basis of the
assumed hkl values. If each entry in this column is identical,
then the entire process is validated.
2θ
Sin2θ
0.143
Sin2θ/Sin2θ1 Clear
(hkl)?
fractions
1.00
3
111
sin2θ
/(h2+k2+l2)
0.0477
44.48
51.83
0.191
1.34
4
200
0.0478
76.35
0.382
2.67
8
220
0.0478
92.90
0.525
3.67
11
311
0.0477
98.40
0.573
4.01
12
222
0.0478
121.87
0.764
5.34
16
400
0.0477
144.54
0.907
6.34
19
420
0.0477
A father-son team that shared a Nobel
Prize
William Henry Bragg (1862–1942),
William Lawrence Bragg (1890–1971)
Nobel Prize (1915)
h2 + k2 + l2
SC
1
100
2
110
3
111
111
4
200
200
5
210
6
211
FCC
BCC
DC
110
111
200
211
7
8
220
220
9
300, 221
10
310
11
311
311
12
222
222
13
320
14
321
220
220
310
311
222
321
15
16
400
17
410, 322
18
411, 330
19
331
400
400
400
411, 330
331
331
Two equivalent ways of stating Bragg’s Law
n 2dhkl sin
1st Form
d hkl
2
sin
n
d nh,nk ,nl
d hkl
2
2
2
n
(nh) (nk) (nl)
a
2dnh nk nl sin
2nd Form
X-rays
Characteristic Radiation, K
Target
Mo
Cu
Co
Fe
Cr
Wavelength, Å
0.71
1.54
1.79
1.94
2.29