Chapter 7
advertisement

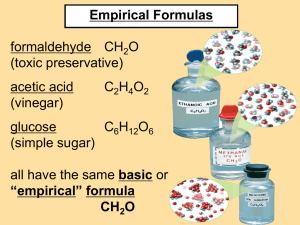
Chapter 7 Chemical Formulas & Chemical Compounds 7.1 Chemical Names & Formulas Ions • Cation: A positive ion • Mg2+, NH4+ • Anion: A negative ion • Cl-, SO42• Ionic Bonding: Force of attraction between oppositely charged ions. Predicting Ionic Charges Groups 3 - 12: Iron (II) = Fe2+ Iron (III) = Fe3+ Many transition elements have more than one possible oxidation state. Predicting Ionic Charges Groups 3 - 12: Zinc = Zn2+ Silver = Ag1+ Some transition elements have only one possible oxidation state. Formula Writing for Binary Ionic Compounds criss-cross the oxidation numbers to balance out the charge. Magnesium Bromide Mg+ 2 Br – 1 Calcium Sulfide Ca + 2 S –2 Mg1Br2 Ca2S2 MgBr2 CaS Writing Ionic Compound Formulas Example: Iron (III) chloride 1. Write the formulas for the cation and anion, including CHARGES! 2. Check to see if charges are balanced. 3. Balance charges, if necessary, using subscripts. Fe3+ Cl- 3 Not balanced! Naming Ionic Compounds 1. Cation first, then anion 2. Monatomic cation = name of the element • Ca2+ = calcium ion 3. Monatomic anion = root + -ide • Cl- = chloride • CaCl2 = calcium chloride Naming Ionic Compounds Metals with multiple oxidation states • some metal forms more than one cation • use Roman numeral in name • PbCl2 • Pb2+ is cation • PbCl2 = lead (II) chloride Elements with Multiple Oxidation Numbers Copper I Copper II Iron II Iron III Mercury I Mercury II Lead II Lead IV Tin II Tin IV Chromium II Chromium III Chromium VI Cu+1 Cu+2 Fe+2 Fe+3 Hg+1 Hg+2 Pb+2 Pb+4 Sn+2 Sn+4 Cr+2 Cr+3 Cr+6 Manganese II Manganese III Manganese VII Cobalt II Cobalt III Gold I Gold III Nickel II Nickel III Nickel IV **Silver Ag+1 **Zinc Zn+2 **Cadmium Cd+2 Mn+2 Mn+3 Mn+7 Co+2 Co+3 Au+1 Au+3 Ni+2 Ni+3 Ni+4 ♥ Poly Atomic Ions to Know and Love ♥ Name Formula Name Formula Hypochlorite ClO-1 Acetate C2H3O2-1 (CH3COO -1) Dichromate Cr2O7-2 Chlorite ClO2-1 Ammonium NH4+1 Chlorate ClO3-1 Nitrate NO3-1 Perchlorate ClO4-1 Nitrite NO2-1 Cyanide CN-1 Hydroxide OH-1 Carbonate CO3-2 Phosphate PO4-3 Chromate CrO4-2 ♥ More Poly Atomic Ions to Know and Love ♥ Name Formula Name Formula Sulfite SO3-2 Hydrogen HCO3-1 Sulfate SO4-2 Hydrogen Sulfite Permanganate HSO3-1 MnO4-1 Carbonate Hydrogen Phosphate Hydrogen Sulfate Oxalate Hydronium H3O+ Silicate SiO3-2 Peroxide O2-2 Phosphite PO3-3 Bromate BrO3-1 Arsenate AsO4-2 HPO4-2 HSO4-1 C2O4-2 Naming Compounds with Polyatomic Ions • • • • Formula (NH4)2SO4 ZnCO3 NH4Br Li2CO3 Name ammonium sulfate zinc carbonate ammonium bromide lithium carbonate * Polyatomic & monatomic cation names remain the same, monatomic anions change their ending to –ide. Writing Ionic Compound Formulas Example: Barium nitrate 1. Write the formulas for the cation and anion, including CHARGES! 2. Check to see if charges are balanced. 3. Balance charges , if necessary, using subscripts. Use parentheses if you need more than one of a polyatomic ion. Ba2+ ( NO3-) Not balanced! 2 Writing Ionic Compound Formulas Example: Ammonium sulfate 1. Write the formulas for the cation and anion, including CHARGES! 2. Check to see if charges are balanced. 3. Balance charges , if necessary, using subscripts. Use parentheses if you need more than one of a polyatomic ion. ( NH4+) 2 SO42- Not balanced! Writing Ionic Compound Formulas Example: Aluminum sulfide 1. Write the formulas for the cation and anion, including CHARGES! 2. Check to see if charges are balanced. 3. Balance charges , if necessary, using subscripts. Use parentheses if you need more than one of a polyatomic ion. Al3+2 S2- 3 Not balanced! Writing Ionic Compound Formulas Example: Magnesium carbonate Mg2+ CO32- 1. Write the formulas for the cation and anion, including CHARGES! 2. Check to see if charges are balanced. 3. Simplify to a formula unit. They are balanced! Writing Ionic Compound Formulas Example: Zinc hydroxide 1. Write the formulas for the cation and anion, including CHARGES! 2. Check to see if charges are balanced. 3. Balance charges , if necessary, using subscripts. Use parentheses if you need more than one of a polyatomic ion. Zn2+ ( OH- ) 2 Not balanced! Writing Ionic Compound Formulas Example: Aluminum phosphate Al3+ PO43- 1. Write the formulas for the cation and anion, including CHARGES! 2. Check to see if charges are balanced. They ARE balanced! More Examples… Chemical Formula 1. 2. 3. 4. 5. 6. 7. Cr2O3 Cr2O CuSO4 Ni(OH)2 Cr2(C2O4)3 Cu2S CuS Chemical Name 1. 2. 3. 4. 5. 6. 7. chromium (III) oxide chromium (I) oxide copper (II) sulfate nickel (II) hydroxide chromium (III) oxalate copper (I) sulfide copper (II) sulfide Hydrates • Hydrate – when a water molecule (s) are chemically bonded to the ionic compound. • Normal ionic naming protocol are used, then followed by the word “hydrate.” • Prefixes are added to indicate the number of water molecules when naming hydrates. Hydrate Prefixes # of water molecules 1 prefix mono- # of water molecules 6 prefix hexa- 2 di- 7 hepta- 3 tri- 8 octa- 4 tetra- 9 nona- 5 penta- 10 deca- Hydrates • Example: MgBr2 ∙ 6H2O Magnesium bromide hexahydrate • The “ ∙ ” means “loosely bonded” • Hygroscopic - easily absorb water molecules from the air. • Deliquescent- very hygroscopic; takes out water from the air to dissolve completely to form a liquid solution. • Anhydrous – when all of the water has been removed. Naming Binary Covalent Compounds • • • • • Compounds between two nonmetals First element in the formula is named first. Second element is named as if it were an anion. Use prefixes Only use mono on second element P2O5 = diphosphorus pentoxide CO2 = carbon dioxide CO = carbon monoxide N2O = dinitrogen monoxide Acids • Acids always begin with Hydrogen Anion Formula Name Cl-1 HCl Hydrochloric Acid Br-1 HBr Hydrobromic Acid SO4-2 H2SO4 Sulfuric Acid SO3-2 H2SO3 Sulfurous Acid NO3-1 HNO3 Nitric Acid CN-1 HCN Hydrocyanic Acid PO4-3 H3PO4 Phosphoric Acid Bases Cation Formula Name Na+1 NaOH Sodium Hydroxide K+1 KOH Potassium Hydroxide NH4+1 NH3 Ammonia Organic Compounds • Organic compounds are named using a different set of rules. • The simplest group is the hydrocarbons. These compounds are composed solely of the elements carbon and hydrogen. • Carbon atoms can link to each other in chains and in rings. Naming Hydrocarbons • The stem of the compound name is then chosen from the following table: # of carbon atoms 1 prefix meth- # of carbon atoms 6 prefix hexa- 2 eth- 7 hepta- 3 prop- 8 octa- 4 but- 9 nona- 5 penta- 10 deca- Hydrocarbons: Alkanes • These molecules have the generic formula: CnH2n+2 • They contain all single bonds. CH4 C2H6 C3H8 C4H10 C5H12 C6H14 methane ethane propane butane pentane hexane Hydrocarbons: Alkenes • These molecules have the generic formula: CnH2n • They contain double bonds between carbon atoms. C2H4 C3H6 C4H8 C5H10 C6H12 ethene propene butene pentene hexene Hydrocarbons: Alkynes • These molecules have the generic formula: Cn Hn • They contain triple bonds between carbon atoms. C2H2 C3H3 C4H4 C5H5 C6H6 ethyne propyne butyne pentyne hexyne Chapter 7 Chemical Formulas & Chemical Compounds 7.2 Oxidation Numbers Oxidation Numbers • Oxidation Number – numbers assigned to atoms composing a compound or ion that indicate the general distribution of electrons among bonded atoms Chapter 7 Chemical Formulas & Chemical Compounds 7.3 Using Chemical Formulas Molar Mass • The mass of 1 mole of a pure substance is called its Molar Mass. • Ex: Molar mass of Iron is 55.847 g/mol What is the molar mass of Platinum? 195.08 g/mol Molar Mass • The molar mass depends on the particles that compose the compound. If your element exists as a molecule, i.e. BrINClHOF, one mole of these particles contains 2 moles of the element as an atom. • Determine the molar mass of oxygen molecules (O2) (16.00 g/mol) x (2 atoms) = 32.00 g/mol The molar mass of oxygen molecules (O2) is twice the molar mass of oxygen atoms! Formula Mass • The molar mass of a compound is the mass of the atomic mass units of one molecule. • This takes into consideration the number of atoms of each element in a compound. • Formula Mass is calculated the same way as molar mass except it is measured in amu, instead of g/mol. Calculating Formula Mass Calculate the formula mass of magnesium carbonate, MgCO3. 24.31 + 12.01 + 3(16.00) = 84.32 amu Steps for Calculating Molar Mass for Compounds 1. List the elements 2. Determine how many atoms of each 3. Identify the atomic masses from the periodic table 4. Multiply how many atoms by the respective atomic mass 5. Add up the totals for the Molar Mass Practice • H2O • NaCl H 2 x 1.008 = 2.016 Na 1 x 22.9 = 22.9 O 1 x 15.99 = 15.99 Cl 1 x 35.45 = 35.45 18.006 g/mol 58.35 • C6H12O6 C 6 x 12.01 = 72.06 H 12 x 1.008 = 12.096 O 6 x 15.99 = 95.94 180.096 g/mol • K2O K 2 x 39.1 = 78.2 O 1 x 15.99 = 15.99 94.19 g/mol g/mol Calculating Percentage Composition Calculate the percentage composition of magnesium carbonate, MgCO3. 24.31 + 12.01 + 3(16.00) = 84.32 amu 24.31 Mg 100 28.83% 84.32 12.01 C 100 14.24% 84.32 48.00 O 100 56.93% 84.32 100.00 Mass Percent • So…. In one mole of H2O, how many grams of Hydrogen are there? 2 mol H x 1.008g H = 2.016 g H in 1 mol H2O 1 mol H • What % of Hydrogen, by mass, is in H2O? 2.016 g H 18 g H20 x 100 = 11.2 % H *Must also find molar mass of H2O What % of Oxygen, by mass is in H2O? Formulas Empirical formula: the lowest whole number ratio of atoms in a compound. Molecular formula: the true number of atoms of each element in the formula of a compound. molecular formula = (empirical formula)n [n = integer] molecular formula = C6H6 = (CH)6 empirical formula = CH Formulas Formulas for ionic compounds are ALWAYS empirical (lowest whole number ratio). Often, these are called formula units. Examples: NaCl MgCl2 Al2(SO4)3 K2CO3 Formulas Formulas for molecular compounds MIGHT be empirical (lowest whole number ratio). Molecular: H2 O C6H12O6 C12H22O11 Empirical: H2O CH2O C12H22O11 Chapter 7 Chemical Formulas & Chemical Compounds 7.4 Determining Chemical Formulas Empirical Formula Determination 1. Base calculation on assumption of 100 grams of compound. 2. Determine moles of each element in 100 grams of compound. 3. Divide each value of moles by the smallest of the values. 4. Multiply each number by an integer to obtain all whole numbers. Empirical Formula Determination Adipic acid contains 49.32% C, 43.84% O, and 6.85% H by mass. What is the empirical formula of adipic acid? 49.32 g C 1 mol C 4.107 mol C 12.01 g C 6.85 g H 1 mol H 6.78 mol H 1.01 g H 43.84 g O 1 mol O 2.74 mol O 16.00 g O Empirical Formula Determination (part 2) Divide each value of moles by the smallest of the values. 4.107 mol C Carbon: 1.50 2.74 mol O 6.78 mol H Hydrogen: 2.47 2.74 mol O 2.74 mol O Oxygen: 1.00 2.74 mol O Empirical Formula Determination (part 3) Multiply each number by an integer to obtain all whole numbers. Carbon: 1.50 x 2 3 Hydrogen: 2.50 x 2 5 Oxygen: 1.00 x 2 2 Empirical formula: C3H5O2 Finding the Molecular Formula The empirical formula for adipic acid is C3H5O2. The molecular mass of adipic acid is 146 g/mol. What is the molecular formula of adipic acid? 1. Find the formula mass of C3H5O2 3(12.01 g) + 5(1.01) + 2(16.00) = 73.08 g Finding the Molecular Formula The empirical formula for adipic acid is C3H5O2. The molecular mass of adipic acid is 146 g/mol. What is the molecular formula of adipic acid? 2. Divide the molecular mass by the mass given by the emipirical formula. 3(12.01 g) + 5(1.01) + 2(16.00) = 73.08 g 146 2 73 Finding the Molecular Formula The empirical formula for adipic acid is C3H5O2. The molecular mass of adipic acid is 146 g/mol. What is the molecular formula of adipic acid? 3. Multiply the empirical formula by this number to get the molecular formula. 3(12.01 g) + 5(1.01) + 2(16.00) = 73.08 g 146 2 73 (C3H5O2) x 2 = C6H10O4 Determining Chemical Formulas from Mass Percents A sample has been analyzed, here are the results: 18.8 % Na 29.0 % Cl 52.2 % O • How can you determine the chemical formula? • Step 1: Assume a 100 g sample. Then, your percent quantities become gram (mass) quantities. 18.8 g Na , 29.0 g Cl & 52.2 g O • Step 2: Convert those masses to moles. 18.8 g Na x 1 mol Na = 0.817 mol Na 23 g Na 29.0 g Cl x 1 mol Cl = 0.817 mol Cl 35.5 g Cl 52.2 g O x 1 mol O = 3.26 mol O 16 g O • Step 3: Since your empirical formula is in small, whole number ratios, divide your mole amounts by the smallest mole quantity. 0.817 mol Na / 0.817 = 1.00 mol Na 0.817 mol Cl / 0.817 = 1.00 mol Cl 3.26 mol O / 0.817 = 3.99 ≈ 4.00 mol O • Step 4: Use these values as subscripts in your formula Na1Cl1O4 ≈ NaClO4 • Step 5: In the event the chemical formula is not the same as the empirical formula, you need the molar mass of the desired compound and you must compare it to the molar mass of the empirical formula. • Step 6: Divide the given molar mass by the empirical molar mass to get the multiple quantity. • Step 7: Multiply each subscript in the formula by that multiple quantity. • Ex: MM of molecular formula = 180 g/mol Using steps 1-4, you found that the empirical formula is CH2O. Find the molar mass of the empirical formula: MM EF = 30 g/mol Divide MM MF / MM EF to get a whole number. Ex: 180 / 30 = 6 C1x6 H2x6 O1x6 C6H12O6 Practice Problems: • A sample has been analyzed to be 10.04 % C, 0.84 % H & 89.12% Cl. Find the Empirical Formula. • A compound’s empirical formula has been determined to be HF. The compound’s molar mass is 40 g/mol. What is its chemical formula? • A compound’s empirical formula has been determined to be CH2. The compound’s molar mass is 42 g/mol. What is its chemical formula?