alkene structure, naming, stereochemistry & preparation
advertisement

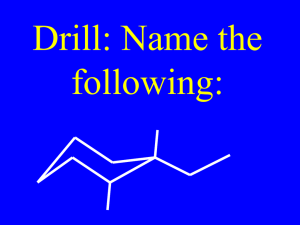
ORGANIC CHEMISTRY 1 Chapter 7 – instructor version ALKENES and CYCLOALKENES • Formulas & naming • Bonding & molecular structure • Physical & chemical properties • Preparation & use • Elimination rxn: E1 and E2 Based on Organic Chemistry, by L.G. Wade, 7th ed; Ch. 7 Prepared by: Dr. Peter Ilich, St. John’s University Queens, New York, Spring 2012 [7.1 & 7.4] Definition & naming: - An alkEne has one or more pairs of doubly bonded carbon atoms, C=C, in its skeleton formula: name: H2C=CH2 H2C=CH2-CH3 H2C=CH2-CH2-CH3 ethene (ethylene) propene butene (or 1-butene) But – watch: H3C-CH2=CH2-CH3 2-butene [7.4] Alkene naming - practice formula: name: H2C=CH2CH2CH2CH3 CH3CH2=CH2CH2CH3 pentene (1-pentene) 2-pentene C atom # 2 CH3-CH2-CH2-H2C=CH2-CH2-CH3 3-heptene C atom # 3, not # 4 [7.4] Naming alkenes – more practice: Formula, condensed: Expand it: Name this compound: Or draw the C-skeleton: 2, not 4 5 Label the position of double bond (smallest number, 2), identify the substituent group and label its position (5): 5-methyl-2-hexene (“old” IUPAC) (or, new IUPAC: 5-methylhex-2-ene) [7.4] The rules for naming alkenes; - Longest chain – the amended rule Name this alkene: 1st – expand it: Not the longest chain, but the longest (and most branched) chain which contains all C=C 1 2 2nd - label the C-skeleton: 5 1 6 9 6 3 2-butyl-3-methylhex-1-ene [7.4] Alkene naming continued: What do you name alkenes with 2 or more C=C? 1,3-pentadiene Name this: Note: 1,3-butadiene Make a note: Allene Name it: 1,3-pentadiene 1,3,7-octatriene But not: 1,2-butadiene Allene is not a diene; it is not even an alkene [7.4] Alkene naming – cycloalkenes Formula 1: Formula 2: Name 1: cyclopropene Name 2: 1,4-cycloheptadiene methyleneRadical group naming: methyl- 1-methylene-3-methylcyclopentane Make a note: No designation of alkene C-atoms as 1º, 2º, 3º, & 4º, as in alkanes 3º C not a 2º C 2º C not a 4º C not a 3º C 4º C [7.4] Alkene naming – practice: (A) (B) (C) [7.2] Ethene electronic structure & geometry: [7.2] El. structure & geo – comparison with alkanes [7.7] Heats of hydrogenation, ΔHH2º - Terminal vs. central C=C bond ΔHH2º [kJ mol-1] -113 3-methyl-1-butene 2-methylbutane -127 2-methyl-2-butene 2-methylbutane NOTE: The lower (more negative) the ΔHH2º number the less stable the alkene. ΔHH2º of alkenes – what do they mean? - Think of standard heats of formation, ΔHfº ΔHfº H2(gas), C(soot) = 0 kJ mol-1 [Ch. 7, JBS 6th ed.] E N less stable more stable E R G -113 kJ -127 kJ Y [ΔHfº (2-MeBu)= -182 kJ mol-1] [7.2] Bonding & molecular properties of alkenes Alkene substitution & stability LESS STABLE MORE STABLE MOST STABLE And – the “take-home message” is: The more substituted (or the fewer H atoms there are on) the C=C atoms the more stable the alkene. [7.5] Alkenes – 2-D structure & isomerism - The C=C part of an alkene is confined and locked into a plane: ΔE = +264 kJ/mol You may not twist an alkene – like, for example, an ethane molecule, which twists ~ 1014 times a second at room temperature – unless you “pay” 264 kJ/mol and break the π – bond. As a consequence, alkenes exist as 2-D isomers, as “E” (Germ., entgegen = oposite) and “Z” (Germ., zusamen = together) forms C,C bond properties: alkenes vs. alkanes (2Z)-butene butane, gauche- (2E)-butene butane, anti- [7.5] Alkenes – 2-D stereochemistry - The cis-/trans-notation for 2-butene, CH3CH=CHCH3 2-D formula: trans-2-butene (methyls opposite) cis-2-butene (methyls together) 2-D formula: Practice: trans-2-pentene or (2E)-pentene [7.5] Alkene 2-D stereochemistry: - The cis-/trans- notation for alkenes is being replaced by E(trans)-/Z(cis)- notation: 1st: Expand the C=C fragment and dissect it vertically 2nd: Identify and rank the groups at left; use the Sequence Rule 5th: Name the compound: 3rd: Do the same for the groups at right 4th: Compare the positions of the first-ranked groups: (Z)- for the same, (E)for the opposite (Z)-3-chloropent-2-ene [7.5] Alkene 2-D stereo – practice Formula: Partial name, specify E/Z: 3-methyl-(3 )-hexene 5-methyl-(2 ,4 )-heptadiene 5-methyl-(1 ,3 )-cycloheptadiene (1 ,3 )-decadiene (A) Biologically important E-/Z-stereomers: Oleic acid C18:1(9c) & C18:1(9t) C18:1(9c) – cis-oleic acid, the common form of oleic acid which can be metabolized: C18:1(9t) – trans-oleic acid: industrially prepared form of oleic acid (through partial hydrogenation); cannot be metabolized and forms insoluble deposits in blood vessels: (B) Human/vertebrate visual pigment – retinal (retinimine) The retinal C11 cis- to trans-isomerization is the physical-chemical basis of our ability to see light The experimentally determined structure of (Rhod)opsin [ Palczewski et al., Science, 289, Issue 5480, 739-745] Impact of light on rhodopsin in our retinas induces retinal-opsin to isomerize at C11 from cis- to trans-form: Trans-retinal separates from the opsin protein and this event triggers within 10-3 [s] a cascade of signals: And – this is how you see: [7.8] Macroscopic physical properties of alkenes - Lower alkenes (C2 to C5) are gases @ R.T. - Boiling point increases with C- number - Boiling point within the same size alkenes decreases with branching (same as alkanes) - Less dense than water (Gulf oil slick!); mixes poorly or not at all with water - More polarizable – C=C bond – than alkanes; induced dipole – induced dipole interactions: they become slightly dipolar in electric field [7.9] Preparation of alkenes: - By elimination reactions - Examples: - E1 elimination of alkyl alcohols - E1 elimination of haloalkanes - E2 elimination of haloalkanes - Other elimination reactions [7.9] Preparation of alkene by elimination; E1 dehydration rxn of alcohols, recapitulation: Acidification & loss of water; note Δ: Carbocation rearrangement (if possible): β-elimination; Zaytsev regioselectivity: [4] Preparation of alkenes by E2 rxn: E2 elimination of haloalkanes: Substrate: usually hindered Reagent: very strong, bulky base Reaction flow: concerted Transition state: concerted reaction Stereochemistry: anti- and syn-periplanar [4] Preparation of alkenes by E2 reactions Example: E2 – continued: When nucleophile, Nu, in an SN2 reaction is also a strong Broensted base, it can lead to a concerted elimination, or the so-called E2 reaction, with the following mechanism: Reaction type: ELIMINATION, E RXN rate = krate * [substrate]*[base] =2nd order, E2 Regiochemistry (where it occurs) of E2 reactions: Zaytsev, E1 (E2) Hoffmann, E2 Make a note - similarities & differences: more stable less stable Elimination regioselectivity: Zaytsev Hoffmann E2 stereoselectivity – the definition: E2 substrate stereochemistry – another view: E2 – the substrate & the product stereochemistry meso-1,2-dibromo-1,2-diphenylethane (E)-1-Bromo-1,2-diphenylethene E2 – stereoselectivity (Important!): Practice: Good E2 geometry: Bad E2 geometry: E2 practice: complete the reaction & name the product: Summary of Ch. 7 – what have we learned today?