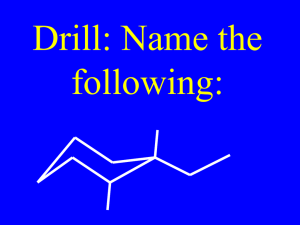
CH 2

Chapter 4
Reactions of Alkenes and Alkynes
1. The most important reaction of alkenes is the addition to the C=C double-bond of various reagents X-Y to yield saturated products
2. A second characteristic reaction of alkenes is the formation of chain-growth polymers
Reactions of alkenes
Electrophilic addition reactions
• Addition of HX ( Hydrohalogenation)
• Addition of H
2
O
• Addition of X
2
• Addition of H
2
• Hydroxylation with KMnO
4
• Oxidative cleavage of alkenes with acidic
KMnO
4
• Polymerization of alkenes
Addition of HX to Alkenes:
Hydrohalogenation
The of halogen acids, HX , to alkenes is a general reaction that allows chemists to prepare a variety of halo-substituted alkane products
• A regio specific reaction: The reactions are regiospecific (regioselective) when only one of two possible directions of addition occurs
CH
3
Cl H H Cl
| | | |
— C — CH
2
CH
3
— C
| |
— CH
2
CH
3
CH
3
Orientation of Alkene Addition Reactions:
Markovnilov ’ s Rule
• In the addition of HX to an alkene, the H attaches to the carbon with fewer alkyl substituents , and the X attaches to the carbon with more alkyl substituents
• Electrophile; H +
Carbocation Structure and Stability
• The electronic structure of a carbocation
1.
Bond angles about the positively charged carbon are 120 °
2.
Carbon uses sp 2 hybrid orbitals to form sigma bonds to the three attached groups
3.
The unhybridized 2p orbital lies perpendicular to the sigma bond framework and contains no electrons
• More highly substituted carbocation are more stable
• Alkyl groups tend to donate electrons to the positively charged carbon atom
• The more alkyl groups there are, the more electron donation there is and the more stable the carbocation
Addition of H
2
O to Alkenes: Hydration
• Addition of water is called hydration
• Acid-catalyzed hydration of an alkene is regioselective H adds to the less substituted carbon of the double bond
• Require high temperature and strongly acidic condition
Other methods
Addition of X
2
to Alkenes: Halogenation
• Carried out with either the pure reagents or in an inert solvent such as CCl
4 or CH
2
Cl
2
A test for a double bond Br
2
(red) → no color
Anti stereochemistry
• Stereoselective reaction : a reaction in which a single starting material has the capacity to form two or more stereoisomeric products but forms one of them in greater ammounts
Addition of H
2
to Alkenes:
Hydrogenation
• Most alkenes react with H
2 in the presence of a transition metal catalyst to give alkanes
– commonly used catalysts are Pt, Pd, Ru, and Ni
• The process is called catalytic reduction or catalytic hydrogenation
• Oxidation: the loss of electrons
• Reduction : the gain of electrons
Syn stereochemistry
Oxidation of Alkenes: Epoxidation,
Hydroxylation and Cleavage
• The addition of oxygen
• Alkenes are oxidized to give epoxides on treatment with a peroxyacid, RCOOOH
• Epoxides undergo an acid-catalyzed ring-opening reaction with water (a hydrolysis) to give the corresponding dialcohol, or diol, also called a glycol
• Hyrdoxylation, the addition of an -OH group
• The hydroxylation of the alkene can also be carried out by reaction with potassium permanganate, KMnO
4
, in basic solution
• The reaction occurs with syn stereochemistry and yields a 1,2-dialcohol, or cis diol , product (also called glycol )
• When oxidation of the alkene is carried out with
KMnO
4 in acidic solution, cleavage of the double bond occurs and carbonyl-containing products are obtained
• The double bond carbons
– contain two substutuents: the products are ketone
– contain one substutuent: the products are carboxylic acid
– contain two hydrogens: the products are CO
2
Addition of Radical to Alkenes:
Polymers
• A polymer is a large molecule built up by repetitive bonding together of many smaller molecules (called monomer)
– Cellulose (sugar)
– Proteins (amino acid)
– Nucleic acid (nucleotide)
– Synthetic polymers
• Many simple alkenes undergo rapid polymerization when treated with a small amount of a radical as catalyst
• High pressure (1000-3000 atm)
• High temperature (100-250 ℃ )
(several thousand monomers)
• Radical polymerization of an alkene involves three kinds of steps:
1. Initiation
2. Propagation
3. Termination
In the mechanism, a curved half-arrow , or “ fishhook , ” is used to show the movement of a single electron
Step 1 Initiation: Reaction begins when a few radicals are generated by the catalyst
• Benzoyloxy peroxide is used as initiator, the O-O bond is broken on heating to yield benzoyloxy radicals
• The benzoyloxy radicals then adds to the C=C bond of ethylene to generate a carbon radical
Step 2 Propagation:
• Polymerization occurs when the carbon radical formed in step 1 adds to another ethylene molecule
• Repetition of this step for hundreds or thousands of times builds the polymer chain
Step 3 Termination:
Polymerization eventually stops when a reaction that consumes the radical occurs
Combination of two growing chains is one possible chain-terminating reaction
2 R-CH
2
CH
2
·
→ R-CH
2
CH
2
CH
2
CH
2
-R
Conjugated Dienes
A compound has altering single and double bonds – so-called conjugated compound --
– If the double bonds are well separated in a molecule, they react independently, but they are close together, they may interact with one another
Buta-1,3-diene is a conjugated diene, whereas penta-1,4-diene is a non-conjugated diene with isolated double bonds
• There is an electronic interaction between the two double bonds of a conjugated diene because of p orbital overlap across the central single bond
• This interaction of p orbitals across a single bond gives conjugated dienes some unusual properties
• HX adds to a conjugated diene, mixtures of products are often obtained
• 3-Bromobut-1-ene is the typical Markovnikov product of 1,2-addition , but 1-bromobut-2-ene appears unusual ( 1,4-addition)
• Allylic carbocation
– Next to the double bond
– More stable than nonallylic
Stability of Allylic Carbocations:
Resonance
• All three carbon atoms are sp 2 -hybridized, and each has a p orbital
• The p orbital on the central carbon can overlap equally well with p orbitals on either of the two neighboring carbons
• The two electrons are free to move about over the entire three-orbital array
• The two individual structures of an allylic carbocation are called resonance forms
– The only difference between the resonance forms is the position of the bonding electrons
• The atoms remain in exactly the same place in both resonance forms – connections and 3-D shapes
• An allylic carbocation has a single, unchanging structure called a resonance hybrid that is blend of the two individual forms
• The greater the number of possible resonance forms, the greater the stability – resonance leads to stability
Drawing and Interpreting Resonance Forms
The lengths of the two C-O bonds are identical
The acetate ion is simply a resonance hybrid of the two resonance forms, with both oxygens sharing the p electrons and the negative charge equally
1. Individual resonance forms are imaginary.
─ The real structure is a resonance hybrid of the different resonance forms
2. Resonance forms differ only in the placement of their p or non-bonding electrons
3. Different resonance forms of a substance don ’ t have to be equivalent
1. Resonance forms must be valid Lewis structures and obey normal rules of valency
2. Resonance leads to stability
– The greater the number of resonance forms, the more stable of the substance
• Localized electrons
– restricted to a particular locality
– belong to a single atom or stay in a bond between two atoms
• Delocalized electrons
– not localized on a single atom, nor localized between two atoms
− p or non-bonding electrons can be moved to near atoms (sp 2 atoms)
1. Toward a positive charge
2. Toward a p bond
3. Toward the more electronegative of the atoms (only p electrons)
• A compound with delocalized electrons is said to have resonance
Alkynes and Their Reactions
• Alkynes are hydrocarbons that contain a carboncarbon triple bond
• C ≣ C bond results from the overlap of two sp hybridized carbon atoms and consists of one spsp s bond and two p-p p bonds
• The general formula is C n
H
2n-2
• Alkynes are named by general rules similar to those used for alkanes and alkenes
• The suffix –yne
• Internal alkynes and terminal alkynes
• Compounds containing both double and triple bonds are called en yne s (not ynenes)
• Numbering of the hydrocarbon chain starts from the end nearer the first multiple bond ,whether double or triple
• If there is a choice in numbering, double bond receive lower number than triple bond
• Common names: prefix the substituents on the triple bond to the name “ acetylene ”
IUPAC
CH
3
C≡C H CH
Propyne
3
C≡C CH
3
CH
2
=CH C≡C H
But-2-yne But-1-en-3-yne
Common
Methyl acetylene Dimethyl acetylene Vinyl acetylene
Addition of H
2
• Lindlar ’ s catalyst can be prepared by precipitating palladium on calcium carbonate and treating it with lead acetate and quinoline
• Syn -addition
• Converted into trans alkenes using Na or Li in liquid ammonia
Addition of HX
• Stopped after addition of 1 equivalent of HX
• An excess of HX leads to formation of a dihalide product
Addition of X
2
• Anti-addition
Addition of H
2
O
• The en ol product rearranges to a more stable isomer, a ketone
• A mixture of both possible ketones results when an internal alkyne is hydrated
• Only a single product is formed from reaction of a terminal alkyne
Formation of acetylide anions
• When a terminal alkyne is treated with a strong base such as sodium amide (NaNH
2
), the terminal hydrogen is removed and an acetylide anion is formed
• Acetylide anions are both acidic and nucleophilic
• Acetylide anion react with alkyl halides to subsitute for the halogen and yield a new alkyne product
• It is a very useful method for preparing large alkyne from small alkyne precursors
• Chapter 7