Periodic table Lecture
advertisement

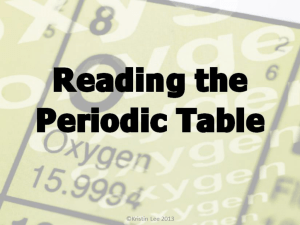
Lec: Periodic Table of Elements 1 1.00794 H Hydrogen Element: Is the simplest form of matter. An atom is the smallest piece of matter that can be identified as an element. 1 1.00794 H Hydrogen Atomic Name: 1 1.00794 H Hydrogen Atomic Number: Indicates the number of protons and electrons in a neutral atom. 1 1.00794 H Hydrogen Atomic Mass: Measured in Atomic Mass Units or AMU’s. 1 AMU is equal to the mass of a proton. 1 1.00794 H Hydrogen Atomic Symbol 1 or 2 letters. If the Atomic symbol has a second letter it must be lower cased. Calculating the number of Neutrons Atomic Mass - Atomic # = # Neutrons (rounded) Ex: Hydrogen 1 - 1 = 0 Hydrogen has no neutrons. Calculate the number of neutrons for O which is oxygen. Oxygen Mass = 15.9994 or 16 Atomic Number = 8 16 - 8 = 8 Oxygen has 8 neutrons. Types of Elements Metals: 1. Good conductors of heat and electricity. 2. Low melting points. 3. Malleable (Easily bent). 4. Shiny or lustrous. Non-Metals: 1. Poor conductors of heat and electricity. 2. Very high melting points. 3. Brittle. 4. Dull. Metalloids: Have characteristics from both metals and non-metals. The following elements are found in the liquid state of matter at room temperature: 35 and 80. These are either a metal or nonmetal. Periods and Groups • Periods are horizontal rows on the Periodic Table of Elements increasing by Atomic Number. • Groups are vertical columns on the Periodic Table of Elements where elements have similar chemical properties. Groups are also called families. On your worksheet complete the table for the element: V Atomic Name: Vanadium Atomic #, # of Protons, and Electrons: 23 # of neutrons: 28 Atomic Mass: 50.9415 amu’s Type of Element: Metal Period 4 Group 5 Atomic Name Origin: English