Atomic Structure
advertisement

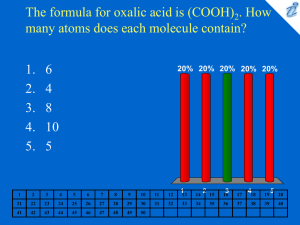
Atomic Structure Subatomic Particles (Particles that make up an atom) ● Proton (p+) - Positively charged - Found in the nucleus - Large mass ● Neutron (n0) - A neutral particle - Found in the nucleus - Large mass ● Electron (e-) - Negatively charged particle - Found outside of the nucleus in the electron cloud - Very small mass Summary ● The nucleus has almost all of the mass & it has a + charge ● The electron cloud has a – charge & creates the atom’s volume How to read a box on the periodic table Atomic # 11 Na Symbol 22.98 ● Atomic Number - # above symbol - Always determines # of protons (can never change!) - Determines # of electrons if atom is neutral (0 charge) - We assume the periodic table is neutral (same # of p+ & e-) ● Summary: - Sodium’s atomic number is 11 - Sodium has 11 protons & 11 electrons 11 Average atomic mass Na 22.98 ● Average atomic mass - # below the symbol in decimal form - The average mass of an atom - Not all sodiums have the same mass due to different number of neutrons (isotope) 11 Na 22.98 Mass # (23) ● Mass Number – rounding the a.a.m. to a whole number - Mass # = # of protons + number of neutrons - Therefore, use to find number of neutrons mass # - # of p = # of n ● Summary - Na’s ave. atomic mass = 22.98 amu (atomic mass units) - Na’s mass # = 23 - Number of neutrons in Na: 23 – 11 = 12 neutrons You just have to try one! Determine: 1. Atomic # = 2. # of protons = 3. # of electrons = 4. Ave. atomic mass = 5. Mass number = 6. # of neutrons = 47 Ag 107.87 Isotopes ● Atoms of the same element can have different numbers of neutrons, therefore, different masses - Remember, neutrons have mass! - Changing the number of neutrons, changes the mass ● Let’s look at 2 isotopes of carbon as an example: - Carbon ALWAYS has 6 protons - But it can have a mass of 12 amu (6p + 6n) C 6 12 or C-12 - and it can have a mass of 14 amu (6p + 8n) 14 6 C or C-14 Perfect practice makes perfect! ● Here is an isotope of oxygen: 18 8 O - How many protons are present? __________ - What is the mass number? __________ - How many neutrons are present? __________ - How many electrons are present? __________ ● Write the shorthand form of a nitrogen isotope that has 13 neutrons. _ _ N or N - __ Mole Conversions ● Moles (mol) are a unit of measurement ● 1 mole = 6.02 x 1023 units (atoms, molecules, formula units, ions, etc) ● 6.02 x 1023 is Avogadro’s number ● Mole Conversions 1 mole = 6.02 x 1023 units = formula weight (grams) What is formula weight? ● Formula weight is the weight of an element or compound in grams ● How is formula weight determined? - Use your periodic table and find the ave. atomic mass - Formula weight of H2O - H’s ave. atomic mass = 1.01 g (x 2) = 2.02 g - O’s ave. atomic mass = 16.00 g 2.02 g + 16.00 g = 18.02 g H2O What is the formula weight of… Al Br2 MgF2 CH4 Ca3(AsO4)2 Conversions 1. Moles to grams # of moles x formula weight (g) = _____ grams 1 1 mole ● Example: How many grams are in 5.00 moles of CaCl2? Formula weight of CaCl2: ● Ca = 40.08 g Cl = 35.45 g (x2) = 70.90 g ● 40.08 g + 70.90 g = 110.98 g CaCl2 5.00 moles x 110.98 g CaCl2 = 554.9 = 555 g CaCl2 1 1 mole 2. Grams to moles # of grams x ___1 mole _ = _______ moles 1 formula wt (g) ● Example: How many moles are in 25.00 g of NaCl? 25.00 g of NaCl x _ 1 mole___ = 0.4278 moles of NaCl 1 58.44 g NaCl 3. Moles to units (atoms, molecules, formula units, ions, etc.) # of moles x 6.02 x 1023 units = ____ units 1 1 mole ● Example: How many atoms are in 0.250 moles of neon? 0.250 moles of Ne 1 x 6.02 x 1023 atoms 1 mole = 1.51 x 1023 atoms of Ne 4. Units to moles # of units x ___1 mole____ = ____ moles 6.02 x 1023 units ● Example: How many moles are in 4.23 x 1024 molecules of H2O? 4.23 x 1024 molecules x ______1 mole______ = 1 6.02 x 1023 molecules 7.03 moles of H2O 1 5. Grams to units # of grams 1 x 6.02 x 1023 units = ____ units formula wt (g) ● Example: How many formula units are in 35.0 g of K2O? 35.0 g K2O x 6.02 x 1023 formula units = 1 94.20 g K2O 2.24 x 1023 formula units of K2O 6. Units to grams # of units 1 x _formula wt (g)_ = ____ grams 6.02 x 1023 units ● Example: How many grams are in 9.75 x 1025 atoms of Ag? 9.75 x 1025 atoms x 1 __107.87 g Ag__ = 17500 g Ag 6.02 x 1023 atoms Mass Percent Composition ● Determining what percentage of each element is in a specific formula ● Example: Find the mass % of each element in NaHCO3. - Step 1: Find their individual ave. atomic masses from the PT & multiply by the number of atoms of each (subscript) Na = 22.99 g (1) H = 1.01 g (1) C = 12.01 g (1) O = 16.00 g (3) = 22.99 g = 1.01 g = 12.01 g = 48.00 g 84.01 g NaHCO3 - Step 2: Add them to get the total weight of the formula. - Step 3: Find the mass % of each! -Remember: Na = 22.99 g (1) H = 1.01 g (1) C = 12.01 g (1) O = 16.00 g (3) = 22.99 g = 1.01 g = 12.01 g = 48.00 g 84.01 g of NaHCO3 - Take the elements individual total weight and divide by the total weight of the formula. Then Multiply by 100. - Mass % of - Mass % of - Mass % of - Mass % of Na = 22.99g /84.01 (100) = 27.36 % H = 1.01g /84.01 (100) = 1.20 % C = 12.01g /84.01 (100) = 14.30 % O = 48.00g /84.01 (100) = 57.14 % - Add %’s to make sure they add up to 100% Getting the formula from mass % ● Do the opposite of finding the mass % ● Example: What is the formula of a substance that is made of 27.29% C & 72.71% O. The total weight of the substance is 44.01 g. - Step 1: Divide each % by 100 then multiply by the total weight C : 27.29/100 = 0.2729 (44.01 g) = 12.01 g C O: 72.71/100 = 0.7271 (44.01 g) = 32.00 g O - Step 2: Divide the totals by their average atomic mass (from PT) 12.01 g C/12.01 g C = 1 32.00 g O/16.00 g O = 2 - Step 3: Put the formula together CO2 Finding the relative atomic mass ● Where does the periodic table get its average atomic masses from? ● Here’s an example: There are two isotopes of chlorine which consists of atoms of relative isotopic masses 35.0 (75.0 %) and 37.0 (25.0 %). % abundance Isotope mass Cl-35 75.0 35.0 amu Cl-37 25.0 37.0 amu (75.0/100) x 35.0 amu + (25.0/100) x 37.0 amu = 35.5 amu The answer matches Cl on the periodic table!