The Mole
advertisement
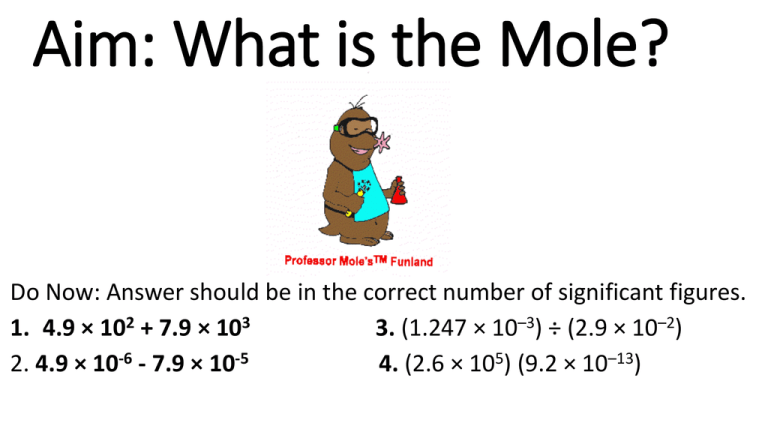
Aim: What is the Mole? Do Now: Answer should be in the correct number of significant figures. 1. 4.9 × 102 + 7.9 × 103 3. (1.247 × 10–3) ÷ (2.9 × 10–2) 2. 4.9 × 10-6 - 7.9 × 10-5 4. (2.6 × 105) (9.2 × 10–13) Counting large numbers… • Easier when you use counting units: Dozen Ream Pair Gross • Regardless of the object, the number that the units represents is always the same • Atoms and molecules are extremely small, making it impossible actually count them (even in the smallest of samples) • Chemists need a unit for counting accurately the # of atoms, molecules, or formula units; they use the mole • Mole (mol) = the SI base unit used to measure the amount of a substance • 1 mole of anything contains 6.02 x 1023 representative particles 1 mole of apples = 6.02 x 1023 apples 1 mole of diamonds = 6.02 x 1023 diamonds 1 mole of baseballs = 6.02 x 1023 baseballs 1 mole of carbon atoms = 6.02 x 1023 carbon atoms The same applies to moles of compounds • How many molecules are in 1 mol NH3? 6.02 x 1023 NH3 molecules • How many molecules are in 1 mol C9H8O4? 6.02 x 1023 C9H8O4 molecules What is Avogadro’s Number • 6.02 X 1023 representative particles/mol • 1 mol of any substance has 6.02 X 1023 representative particles What are representative particles? • Representative particles refers to the species present in a substance: usually atoms, molecules, or formula units Converting Particles to Moles • How many roses are in 3 ½ dozen? USE CONVERSION FACTORS!!! 3.5 dozen x 12 roses =42 roses 1 dozen • How many pencils are in 6 gross? 6 gross x 144 pencils =864 pencils 1 gross conversion factor conversion factor Conversion factor • A conversion factor is used to convert a measured quantity to a different unit of measure without changing the relative amount. To accomplish this, a ratio (fraction) is established that equals one (1) Converting Number of Particles to Mole Conversion factor Converting number of atoms to moles Units you want are on top 0.465 These units cancel out Converting Moles to Particles and Particles to Moles • How many moles contain 4.50 x 1024 atoms of zinc? 4.50 x 1024 atoms Zn x 1 mol zinc 6.02 x 1023 atoms Zn 7.48 mol Zn Solve How many moles of magnesium is 1.25 X 1023 atoms of magnesium? Converting moles to number of particles Conversion factor Converting Moles to Particles How many atoms can be found in 3.91 moles of xenon? Converting Moles to Particles • How many particles of sucrose are in 3.50 moles of sucrose? 3.50 mol sucrose x 6.02 x 1023 rep. part. 1mol 2.11 x 1024 particles of sucrose Solve How many cadmium atoms are there in 6.57 × 103 moles? • 2. How many molecules are in 2.00 moles of H2O? Conv. Factor(s) Set-Up • 3. How many atoms are in 2.00 moles of H2O? Conv. Factor(s) Set-Up • How many moles are in 6.02 x 1023 atoms of carbon? Conv. Factor(s) Set-Up • 10. If you have 0.00812 mole of H2CO3, how many molecules do you have? Conv. Factor(s) Set-Up