kinetic_molecular_theory_and_gases
advertisement

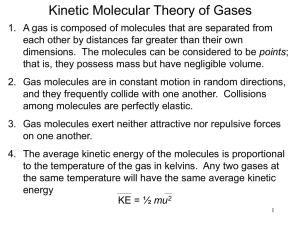
Kinetic Molecular Theory & Gases An Honors/AP Chemistry Presentation Kinetic Molecular Theory Kinetic means motion So the K.M.T. studies the motions of molecules. Solids - vibrate a little Liquids - vibrate, rotate, and translate (a little) Gases - vibrate, rotate, and translate (a lot)! Basic Assumptions of KMT Gases consist of large numbers of molecules in continuous random motion. The volume of the molecules is negligible compared to the total volume. Intermolecular interactions are negligible. When collisions occur, there is a transfer of kinetic energy, but no loss of kinetic energy. The average kinetic energy is proportional to the absolute temperature. Gas Properties Volume - amount of space (L or mL) Temperature - relative amount of molecular motion (K) Pressure - the amount of force molecules exert over a given area (atm, Torr, Pa, psi, mm Hg) Moles - the number of molecules (mol) Temperature Conversions C = 5/9(F-32) F = 9/5C + 32 K = C + 273 So what is the absolute temperature (K) of an object at -40 oF? Answer to Temperature Conversion -40 oF = -40 oC -40 oC = 233 K or 230 K Pressure Conversions QuickTime™ and a decompressor are needed to see this picture. 1 atm = 760 mm Hg = 760 Torr = 101,325 Pa = 14.7 psi How many atmospheres is 12.0 psi? How many Torr is 1.25 atm? How many Pascals is 720 mm Hg? Answers to Pressure Conversions 12.0 psi = .816 atm 1.25 atm =950. Torr 720 mm Hg = 96000 Pa A Barometer A mercury barometer measures air pressure by allowing atmospheric pressure to press on a bath of mercury, forcing mercury up a long tube. The more pressure, the higher the column of mercury. More on the barometer Although American meteorologists will sometimes measure the height in inches, typically this pressure is measured in mm Hg. 1 mm Hg = 1 Torr S.T.P. When making comparisons we often use benchmarks or standards to compare against. In chemistry Standard Temperature is 0 oC (273K) and Standard Pressure is 1 atm. Boyle’s Law If the amount and temperature of the gas are held constant, then the volume of a gas is inversely proportional to the pressure it exerts. Mathematically this means that the pressure times the volume is a constant. P*V = k P1V1=P2V2 Boyle’s Law in Action Sample Questions The volume of a balloon is 852 cm3 when the air pressure is 1.00 atm. What is the volume if the pressure drops to .750 atm? A gas is trapped in a 2.20 liter space beneath a piston exerting 25.0 psi. If the volume expands to 2.75 L, what is the new pressure? The Answers are… P1V1=P2V2 (1atm)(852cm3)= (.750atm)*V2 V2 =1140cm3 (25.0psi)(2.20L)=P2(2.75L) P2 = 20.0 psi Charles’ Law If the amount and the pressure of a gas are held constant, then the volume of a gas is directly proportional to its absolute temperature. Mathematically, this means that the volume divided by the temperature is a constant. V/T = k V1/T1=V2/T2 Charles Law in Action Sample Questions The volume of a balloon is 5.00 L when the temperature is 20.0 oC. If the air is heated to 40.0 oC, what is the new volume? 3.00 L of air are held under a piston at 0.00 oC. If the air is allowed to expand at constant pressure to 4.00 L, what is the new Celsius temperature of the gas? The Answers Are… V1/T1=V2/T2 5.00L/293K = V2/313K V2=5.34L 273K/3.00L = T2/4.00L T2=364K=91oC The Gay-Lussac Law If the amount and volume of the gas are held constant, then the pressure exterted by the gas is directly proportional to its absolute temperature. Mathematically this means that the pressure divided by the temperature is a constant. P/T = k P1/T1=P2/T2 The Gay-Lussac Law in Action Sample Questions A tank of oxygen is stored at 3.00 atm and -20 oC. If the tank is accidentally heated to 80 oC, what is the new pressure in the tank? A piston is trapped in place at a temperature of 25 oC and a pressure of 112 kPa. At what celcius temperature is the pressure 102 kPa? The Answers are… P1/T1=P2/T2 (3atm)/(253 K)= P2/ (353 K) P2 =4.19 atm (298 K)/(112 kPa)=T2/(102kPa) T2 = 271 K = -2 oC Avogadro’s Law If the temperature and the pressure of a gas are held constant, then the volume of a gas is directly proportional to the amount of gas. Mathematically, this means that the volume divided by the # of moles is a constant. V/n = k or V/m = k V1/n1=V2/n2 or V1/m1=V2/m2 Avogadro’s Law in Action Sample Questions The volume of a balloon is 5.00 L when there is .250 mol of air. If 1.25 mol of air is added to the balloon, what is the new volume? 3.00 L of air has a mass of about 4.00 grams. If more air is added so that the volume is now 24.0 L, what is the mass of the air now? The Answers Are… V1/n1=V2/n2 or V1/m1=V2/m2 5.00L/.250 mol = V2/1.50 mol V2=30.0 L 4.00g/3.00L = m2/24.0L m2=32.0 g The Combined Gas Law This law combines the inverse proportion of Boyle’s Law with the direct proportions of Charles’, Gay-Lussac’s, and Avogadro’s Laws. P1V1/(n1T1) = P2V2/(n2T2) or P1V1/T1 = P2V2/T2 Four Gas Laws in One The combined gas law could be used in place of any of the previous 4 gas laws. For example, in Boyle’s Law, we assume that the amount and temperature are constant. So if we cross them off of the combined gas law: P1V1/(n1T1) = P2V2/(n2T2) P1V1 = P2V2 Another Example A sample of hydrogen has a volume of 12.8 liters at 104 oF and 2.40 atm. What is the volume at STP? The answer is: P1V1/(n1T1) = P2V2/(n2T2) P1=2.40atm,V1=12.8L, T1=104oF=40oC=313K, T2=273K, P2=1atm, n1=n2 (2.4atm)(12.8L)/(313K) = (1atm)V2/(273K) V2 = 26.8 L The Ideal Gas Law If, P1V1/(n1T1) = P2V2/(n2T2) Then PV/(nT) = constant That constant is R, the ideal gas law constant. R = .0821 L*atm/(mol*K) R = 8.314 J/(mol*K) So, PV=nRT But what about… Since n = m/M, we can substitute into PV = nRT and get PVM = mRT Since D = m/V, we can substitute in again and get PM = DRT So which one is it? Like a good carpenter, it is good to have many tools so that you can choose the right tool for the right job. If I am solving a gas problem with density, I use PM = DRT. If I am solving a gas problem with moles, I use PV = nRT. If I am solving a gas problem with mass, I use PVM = mRT. Such as…. Under what pressure would oxygen have a density of 8.00 g/L at 300 K? PM = DRT P(32 g/mol) = (8 g/L)(.0821 latm/molK)(300 K) P = 6.16 atm An Important Number What is the volume of 1 mole of a gas at STP? PV = nRT V = nRT/P V = (1mol)*(.0821Latm/molK)(273K)/ (1atm) V = 22.4 L This is called the standard molar volume of an ideal gas. Gas Stoichiometry We had said that stoichiometry implied a ratio of molecules, or moles. Up until now we only used mole ratios. However Avogadro said that the volume is directly proportional to the number of molecules. This means that we can do stoichiometry with volume or moles. Example 1 of Gas Stoichiometry What volume of hydrogen is needed to synthesize 6.00 liters of ammonia? N2 (g) + 3 H2 (g) --> 2 NH3 (g) 6.00 L H2 x (2 NH3/3 H2) = 4.00 L NH3 Example 2 of Gas Stoichiometry What mass of nitrogen is needed to synthesize 20.0 L of ammonia at 1.50 atm and 25 oC? N2 (g) + 3 H2 (g) --> 2 NH3 (g) 20.0 L NH3 x (1 N2/2 NH3) = 10.0 L N2 PVM = mRT (1.5 atm)(10 L)(28 g/mol) = m(.0821Latm/molK)(298K) m = 17.2 g N2 Dalton’s Law When we talk about air pressure, we need to understand that air is not oxygen. Air is a solution of nitrogen (78.09%), oxygen (20.95%), argon (.93%), and CO2 (.03%). So when we talk about air pressure, which gas are we talking about? ALL OF THEM! Dalton’s Law of Partial Pressures states that the total pressure of a system is equal to the sum of the partial (or individual) pressures of each component. Ptotal = P1 + P2 + … Px So if air pressure is 1 atm, then we can assume that the N2 is .78 atm, the O2 is .21 atm, and the Ar is about .01 atm. A Corollary If we extend Boyle’s Law and Avogadro’s Law, we could infer that, at constant temperature and volume, the pressure of a gas is directly proportional to its pressure. P1/Ptotal = n1/ntotal An important example QuickTime™ and a decompressor are needed to see this picture. A sample of CaCO3 is heated, releasing CO2, which is collected over water (a typical practice). The pressure in the collection bottle is the sum of the pressure of the CO2 plus the pressure of the water vapor (since some water always evaporates). Ptotal = PCO2 + PH2O Sample Water Vapor Pressures Water Vapor Pressure Table Temperature Pre ssure (¡C) 0.0 5.0 10.0 12.5 15.0 15.5 16.0 16.5 17.0 17.5 18.0 18.5 19.9 (mmHg) 4.6 6.5 9.2 10.9 12.8 13.2 13.6 14.1 14.5 15.0 15.5 16.0 16.5 Temperature Pre ssure (¡C) 19.5 20.0 20.5 21.0 21.5 22.0 22.5 23.0 23.5 24.0 24.5 25.0 26.0 (mmHg) 17.0 17.5 18.1 18.6 19.2 19.8 20.4 21.1 21.7 22.4 23.1 23.8 25.2 Temperature Pre ssure (¡C) 27.0 28.0 29.0 30.0 35.0 40.0 50.0 60.0 70.0 80.0 90.0 95.0 100.0 (mmHg) 26.7 28.3 30.0 31.8 42.2 55.3 92.5 149.4 233.7 355.1 525.8 633.9 760.0 So in our example If a total pressure of 365 Torr is collected at 25 oC in a 100 ml collection bottle: What is the partial pressure of CO2? What mass of CaCO3 decomposed? Here’s how it works Ptotal = PCO2 + PH2O 365 Torr = PCO2 + 23.8 Torr PCO2 = 341.2 Torr = .449 atm PVM = mRT (.449 atm)(.100 L)(44.0 g/mol) = m(.0821Latm/molK)(298K) m = .0807 g CO2 Corollary Problem A gas collection bottle contains .25 mol of He, .50 mol Ar, and .75 mol of Ne. If the partial pressure of Helium is 200 Torr: What is the total pressure in the system? What are the partial pressures of Ne and Ar? The answers are… nHe = .25 mol, nAr = .50 mol, nNe = .75 mol, PHe = 200 Torr. ntotal =1.50 mol Ptotal/Phe = ntotal/nHe Ptotal/200Torr = 1.50 mol/.25 mol Ptotal = 1200 Torr PAr/Ptotal = nAr/ntotal Par/1200 = .50 mol/1.50 mol PAr = 400 Torr PNe = 1200 Torr - 400 Torr - 200 Torr Pne = 600 Torr Temperature and Kinetic Energy Earlier, I stated that temperature is a relative measure of molecular motion. By definition, Kinetic energy is a measure of the energy of motion. Pretty similar right? Yes they are KEav = 3/2*R*T The average kinetic energy depends only on the absolute temperature. R, the Ideal Gas Law Constant, should be 8.314 J/molK, since we will want the energy in the proper SI unit of Joules. A Thought Question Which of the following ideal gases would have the largest average kinetic energy at 25oC? He, N2, CO, or H2 They are all the same! Since Keav = 3/2*R*T, the mass does not make a difference (ideally). KE = 3/2*(8.314J/molK)*(298K) KE = 3716 J/mol Speed vs Kinetic Energy In physics, you learned that KE = 1/2*m*v2. The velocity, v, describes the speed of an object in a specific direction. If the mass, m, is measured in kg and the velocity is measured in m/s, then the kinetic energy would be measured in Joules. Physics to Chemistry Rewriting the physics version, we could say that v=√(2*KE/m). In chemistry, the Kinetic energy is measured in J/mol, so the mass would have to be measured in Kg/mol which is essentially molar mass. Root Mean Square Speed In Chemistry, we are not worried about velocities in multiple directions. We want an average speed independent of direction. We call this Vrms - the root mean square speed. Vrms = √(3RT/M) A Thought Question Revisited Which of the following ideal gases would have the largest root mean square speed at 25oC? He, N2, CO, or H2 This Time They Are Different Vrms = √(3RT/M) For He, Vrms = √(3RT/M) = √(3*8.314J/molK*298K)/4g/mol = 1363 m/s For N2, Vrms = √(3RT/M) = √(3*8.314J/molK*298K)/28g/mol = 515 m/s Since the molar mass is the same for N2 and CO, their Vrms would be the same, 515 m/s. For H2, Vrms = √(3RT/M) = √(3*8.314J/molK*298K)/2g/mol = 1928 m/s Because H2 is the lightest, it moves the fastest. And this leads us to… QuickTime™ and a decompressor are needed to see this picture. Rate1*√M1 = Rate2*√M2 Graham’s Law The rate of effusion (or diffusion) is inversely proportional to the square root of the molar mass. Effusion is the process of a gas escaping from one container through a small opening. Diffusion is the process of a gas spreading out in a large container. Rate vs. Speed QuickTime™ and a decompressor are needed to see this picture. When we say rate, we are talking about an amount of gas (moles, grams, or even liters) per unit of time. This is not the same as speed which is distance over time. However, the main idea is the same; lighter gases move/effuse faster. For example Under a given set of conditions, oxygen diffuses at 10 L/hr. A different gas diffuses at 20 L/hr under the same conditions. What is the molar mass of this gas? 2 ways to solve this By the equation: Qui ckT ime™ and a dec ompress or are needed to s ee this pic ture. Rate1*√M1 = Rate2*√M2 10 L/hr(√32g/mol) = 40 L/hr *√M2 M2 = 2 g/mol By Logic: If the rate of the unknown gas is 4 times faster, it must be 42, or 16, times lighter. 32 g/mol divided by 16 is 2 g/mol. Real vs. Ideal At the start of the presentation, we talked about the major assumptions of the Kinetic Molecular Theory. If a gas obeys the KMT, it is ideal. If it doesn’t obey the KMT, it is real. So What does that Mean? The molecules of an ideal gas do not interact with one another, except to collide elastically. The molecules of a real gas will interact, to some degree. Since no gases are always ideal, the trick is to make a real gas behave ideally. Real Gases Behaving Ideally If we don’t want the molecules attracting or repelling one another, the first issue is to use a nonpolar gas. If we use smaller amounts of the gas, there are less chances of them interacting. Real Gases Behaving Ideally If we put the gas in larger volumes, the molecules will not interact as much. Likewise, if we keep the gas under low pressure , the molecules will not interact as much. This could also be stated by having molecules that have low densities. Real Gases Behaving Ideally The smaller the molecules, the less likely they are to interact. Lastly, at higher temperatures the molecules are moving too fast to actually interact with one another - they are more likely to collide elastically. Phase Diagrams A phase diagram shows how the different states of matter exist based on the pressure and temperature. A Typical Phase Diagram Water is not Typical… …and Helium is weird!