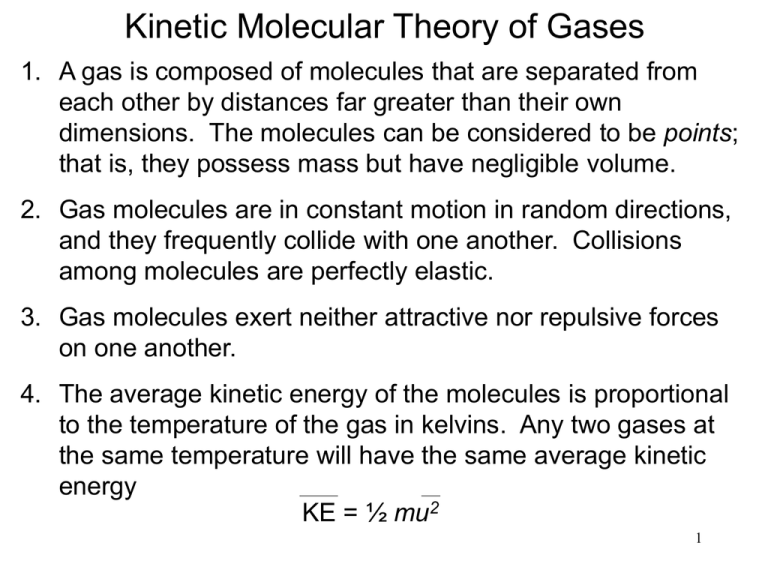
Kinetic Molecular Theory of Gases
1. A gas is composed of molecules that are separated from
each other by distances far greater than their own
dimensions. The molecules can be considered to be points;
that is, they possess mass but have negligible volume.
2. Gas molecules are in constant motion in random directions,
and they frequently collide with one another. Collisions
among molecules are perfectly elastic.
3. Gas molecules exert neither attractive nor repulsive forces
on one another.
4. The average kinetic energy of the molecules is proportional
to the temperature of the gas in kelvins. Any two gases at
the same temperature will have the same average kinetic
energy
KE = ½ mu2
1
Kinetic theory of gases and …
• Compressibility of Gases
• Boyle’s Law
P a collision rate with wall
Collision rate a number density
Number density a 1/V
P a 1/V
• Gay-Lussac’s Law
P a collision rate with wall
Collision rate a average kinetic energy of gas molecules
Average kinetic energy a T
PaT
2
Kinetic theory of gases and …
• Compressibility of Gases
• Charles’s Law
T a average kinetic energy of molecules
Collision rate a average kinetic energy of gas molecules
P a collision rate with wall
…. But! In Charles’s law, pressure stays constant…
To decrease the collision rate, the volume needs to be
increased
VaT
3
Pressure and Volume (Boyle’s Law)
Figure 5.14
The Effects of Decreasing the Volume of a Sample of Gas at Constant Temperature
Decreasing the volume causes the particles to collide with the
walls of the container more frequently, increasing the pressure
Copyright©2000 by Houghton
Mifflin Company. All rights reserved.
4
Pressure and Temperature (Gay-Lussac’ Law)
Figure 5.15
The Effects of Increasing the Temperature of a Sample of Gas at Constant Volume
Increasing the temperature causes the particles to move faster
increasing the frequency of collision with the walls of the
container. If the volume is constant this results in and increase in
pressure
Copyright©2000 by Houghton
Mifflin Company. All rights reserved.
5
Volume and Temperature (Charles’s Law)
Figure 5.16
The Effects of Increasing the Temperature of a Sample of Gas at Constant Pressure
Increasing the temperature causes the particles to move faster
increasing the frequency of collision with the walls of the
container. If the pressure remains constant, the volume of the
container will increase to compensate for the increased motion of
the particles
6
Copyright©2000 by Houghton
Mifflin Company. All rights reserved.
Kinetic theory of gases and …
• Avogadro’s Law
P a collision rate with wall
Collision rate a number density
Number density a n
Pan
• Dalton’s Law of Partial Pressures
Molecules do not attract or repel one another
P exerted by one type of molecule is unaffected by the
presence of another gas
Ptotal = SPi
7
Volume and Number of Moles (Avogadro’s
Law)
Figure 5.17
The Effects of Increasing the Number of Moles of Gas Particles at Constant Temperature and Pressure
Increasing the number of particles at constant temperature and
pressure results in an increase in collisions. The volume of the
container will increase to compensate for the increased number of
collision.
Copyright©2000 by Houghton
Mifflin Company. All rights reserved.
8
KMT – What happens in “real” gases
A gas is composed of molecules that are separated from each
other by distances far greater than their own dimensions. The
molecules can be considered to be points; that is, they possess
mass but have negligible volume.
Gas molecules are in constant motion in random directions,
and they frequently collide with one another. Collisions among
molecules are perfectly elastic.
Gas molecules exert neither attractive nor repulsive forces on
one another.
Under what conditions will gases most
likely exhibit nonideal behavior??
9
Deviations from Ideal Behavior
1 mole of ideal gas
PV = nRT
PV = 1.0
n=
RT
Repulsive Forces
Attractive Forces
10
Effect of intermolecular forces on the pressure exerted by a gas.
11
Van der Waals equation
nonideal gas
}
corrected
pressure
}
2
an
( P + V2 ) (V – nb) = nRT
corrected
volume
Pressure Correction: Takes into account the
probability that a molecule will end up close
enough to another molecule causing an
intermolecular attraction
Volume Correction: Takes into account that
molecules, while extremely small, do take up some
amount of volume
Which element/molecule has the weakest
attraction to each other??
12
Given that 3.50 moles of NH3 occupy 5.20 L at 47˚C, calculate
the pressure of the gas (in atm) using the ideal gas law, and
then the van der Waals equation.
Ideal gas law
Van der Waals Equation
V=5.20 L
V=5.20 L
2 K
T=(47+273.15)
=
3.20x10
T=(47+273.15) = 3.20x102 K
n=3.50 mol
n=3.50 mol
R=0.0821 L·atm/K·mol
2
2
a=4.17 atm·L /mol
nRT
P
b=0.0371 L/mol
2
an2 (4.17 atmL mol 2 )(3.50m ol)
1.89atm
2
2
V
(5.20L)
nb (3.50m ol)(0.0371L mol ) 0.130L
2
V
(3.50m ol)(0.0821Latm K mol )(320K )
P
5.20L
P 17.7atm
an2
( P 2 )(V nb) nRT
V
( P 1.89atm)(5.20L 0.130L) (3.50m ol)(0.081Latm K mol )(320K )
P 16.2atm
13
Homework: Problem 5.89
Calculate the pressure exerted by 2.50 moles of CO2
confined in a volume of 5.00 L at 450 K if the gas is
behaving ideally.
It has been shown that this gas exhibits non-ideal
behavior. Calculate the actual pressure given a=3.59;
b=0.0427.
14
The Meaning of Temperature
(KE)avg
3
RT
2
Kelvin temperature is an index of the random motions
of gas particles (higher T means greater motion.)
Copyright©2000 by Houghton
Mifflin Company. All rights reserved.
15
Kinetic Molecular Theory
• Root mean square speed is an average
molecular speed
• For one mole of a gas KE = 3/2 RT
• For one molecule
KE 1 2 mu 2
KE = kinetic energy
• Therefore
NA
1
R = universal gas constant
T = temperature (in K)
m = mass
u = speed
Bar over top = root mean square average
2
m
u
3 2 RT
2
3RT
u
2
u urms
2
3RT
where R 8.3145J
Copyright©2000 by Houghton
Mifflin Company. All rights reserved.
K m ol
Apparatus for Studying Molecular Speed Distributiona
17
The distribution of speeds
of three different gases
at the same temperature
The distribution of speeds
for nitrogen gas molecules
at three different temperatures
urms =
M
3RT
18
Calculate the root mean squared speed of molecular chlorine in
m/s at 20ºC.
urms
urms
3RT
M
3(8.314 J K mol )(298 K )
0.07090 kg mol
1J 1
kgm 2
s2
urms 321m s
19
Problem 5.78
The temperature in the stratosphere is 23ºC. Calculate the root mean square
speeds of N2 molecules in this region.
N2=____
20
Gas diffusion is the gradual mixing of molecules of one gas
with molecules of another by virtue of their kinetic properties.
r1
r2
=
M2
M1
molecular path
NH4Cl
NH3
17 g/mol
HCl
36 g/mol
21
Gas effusion is the is the process by which gas under
pressure escapes from one compartment of a container to
another by passing through a small opening.
r1
r2
=
t2
t1
=
M2
M1
Nickel forms a gaseous compound of the formula Ni(CO)x What
is the value of x given that under the same conditions methane
(CH4) effuses 3.3 times faster than the compound?
r1 2
x M1 = (3.3)2 x 16 = 174.2
r1 = 3.3 x r2
M2 =
r2
x = 4.1 ~ 4 22
M1 = 16 g/mol
58.7 + x • 28 = 174.2
( )
Justify the statement:
A helium-filled rubber balloon deflates faster
than an air-filled one.
23
Problem 5.83
A gas evolved from the fermentation of
glucose is found to effuse through a
porous barrier in 15.0 min. Under the
same condition of temperature and
pressure, it takes an equal volume of N2
gas 12.0 min to effuse through the same
barrier. Calculate the molar mass of the
gas.
What could this gas be?
24