Chapter 10
advertisement

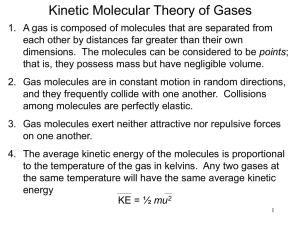
Chapter 10 Gases Barometers and Standard Atmospheric Pressure Barometers and Standard Atmospheric Pressure • Standard atmospheric pressure defined as the pressure sufficient to support a mercury column of 760mm (units of mmHg, or torr). Barometers and Standard Atmospheric Pressure • Standard atmospheric pressure defined as the pressure sufficient to support a mercury column of 760mm (units of mmHg, or torr). • Another unit was introduced to simplify things, the atmosphere (1 atm = 760 mmHg). Barometers and Standard Atmospheric Pressure • Standard atmospheric pressure defined as the pressure sufficient to support a mercury column of 760mm (units of mmHg, or torr). • Another unit was introduced to simplify things, the atmosphere (1 atm = 760 mmHg). • 1 atm = 760 mmHg = 760 torr = 101.325 kPa (page 262). STP standard temperature and pressure Standard temperature 0°C or 273 K Standard pressure 1 atm (or equivalent) – Pressure varies inversely with volume – Volume varies inversely with pressure “The volume of a sample of gas is inversely proportional to its pressure, if temperature remains constant.” Boyle’s Law Boyle’s Law: Pressure & Volume (Figure 10.6 (a) page 263) P V P V Boyle’s Law: Pressure-Volume Relationships A sample of air occupies 73.3 mL at 98.7 atm and 0 ºC. What volume will the air occupy at 4.02 atm and 0 ºC? 1800 mL Boyle’s Law: Pressure-Volume Relationships A sample of helium occupies 535 mL at 988 mmHg and 25 °C. If the sample is transferred to a 1.05-L flask at 25 °C, what will be the gas pressure in the flask? 503 mm Hg • Effects of temperature on a gas • Volume varies directly with Temperature “The volume of a quantity of gas, held at constant pressure, varies directly with the Kelvin temperature.” Charles’s Law a Charles Law: Volume and Temperature (Figure 10.8 Page 266) Charles’ Law and Absolute Zero •Extrapolation to zero volume gives a temperature of -273°C or 0 K Charles’s Law: Temperature-Volume Relationships A sample of oxygen gas occupies a volume of 2.10 L at 25 °C. What volume will this sample occupy at 150 °C? (Assume no change in pressure.) 2.98 L Charles’s Law: Temperature-Volume Relationships A sample of oxygen gas occupies a volume of 2.10 L at 25 °C. At what Celsius temperature will the volume of oxygen occupy 0.750 L? (Assume no change in pressure.) -167°C Pressure vs. Temperature • Pressure varies directly with Temperature • If the temperature of a fixed volume of gas doubles its pressure doubles. Pressure vs. Temperature • The pressure exerted by a gas is directly related to the Kelvin temperature. • V is constant. Pressure vs. Temperature Example A gas has a pressure of 645 torr at 128°C. What is the temperature in Celsius if the pressure increases to 1.50 atm? Pi = 645 torr Ti = 128°C + 273 = 401 K Pf = 1.50 atm 760 torr = 1140 torr 1 atm Tf = ?K Solution T2 = 401 K x 1140 torr = 709K 645 torr 709K - 273 = 436°C Combined Gas Law Problem A sample of helium gas has a volume of 0.180 L, a pressure of 0.800 atm and a temperature of 29°C. What is the new temperature(°C) of the gas at a volume of 90.0 mL and a pressure of 3.20 atm? Combined Gas Law Problem A sample of helium gas has a volume of 0.180 L, a pressure of 0.800 atm and a temperature of 29°C. What is the new temperature(°C) of the gas at a volume of 90.0 mL and a pressure of 3.20 atm? 302 K x 3.20 atm 0.800 atm x 90.0 mL = 604 K 180.0 mL 604 K - 273 = 331 °C Combined Gas Law • A 10.0 cm3 volume of gas measured 75.6 kPa and 60.0C is to be corrected to correspond to the volume it would occupy at STP. 6.12 cm3 Gay-Lussac’s Law Gay-Lussac’s Law of combining volumes: at a given temperature and pressure, the volumes of gases which react are ratios of small whole numbers. How many liters of steam can be formed from 8.60L of oxygen gas? 17.2 L How many liters of hydrogen gas will react with 1L of nitrogen gas to form ammonia gas? 3L H2 A A How many mL of hydrogen are needed to produce 13.98 mL of ammonia? 20.97 ml NH3 Avogadro’s Law: Equal volumes of gases at the same temperature and pressure contain the same number of particles. The molar volume of a gas at STP = 22.4L 22.4 L Ideal Gas • An ideal gas is defined as one for which both the volume of molecules and forces of attraction between the molecules are so small that they have no effect on the behavior of the gas. Ideal Gas Equation PV=nRT R values • A a • I •R values for atm and kPa on Page 272 in book. Calculate the volume occupied by 0.845 mol of nitrogen gas at a pressure of 1.37 atm and a temperature of 315 K. 15.9 L Find the pressure in millimeters of mercury of a 0.154 g sample of helium gas at 32°C and contained in a 648 mL container. 1130 mm Hg An experiment shows that a 113 mL gas sample has a mass of 0.171 g at a pressure of 721 mm Hg and a temperature of 32°C. What is the molar mass (molecular weight) of the gas? 40.0 g/mol Can the ideal “gas” equation be used to determine the molar mass of a liquid? Homework • Do the lab summary for “The Molecular Mass of a Volatile Liquid”. It is due ____. • Attempt the pre-lab for “The Molecular Mass of a Volatile Liquid”. It is due ____. Problem: A volatile liquid is placed in a flask whose volume is 590.0 ml and allowed to boil until all of the liquid is gone, and only vapor fills the flask at a temperature of 100.0 oC and 736 mm Hg pressure. If the mass of the flask before and after the experiment was 148.375g and 149.457 g, what is the molar mass of the liquid? 57.9 g/mol What is the density of methane gas (natural gas), CH4, at 125oC and 3.50 atm? 1.71 g/L Calculate the density in g/L of O2 gas at STP. 1.43 g/L Dalton’s Law of Partial Pressure • The total pressure in a container is the sum of the partial pressures of all the gases in the container. • In a gaseous mixture, a gas’s partial pressure is the one the gas would exert if it were by itself in the container. • Ptotal = P1 + P2 + P3 • Ptotal = 100 KPa + 250 KPa + 200 KPa = 550 KPa Two 1.0 L containers, A and B, contain gases with 2.0 atm and 4.0 atm, respectively. Both gases are forced into Container B. Find the total pressure of the gas mixture in B. A B P V A 2.0 atm 1.0 L B 4.0 atm 1.0 L Vmixture P 2.0 atm 1.0 L 4.0 atm Total = 6.0 atm Dalton’s Law Problem • Air contains oxygen, nitrogen, carbon dioxide, and trace amounts of other gases. What is the partial pressure of oxygen at standard conditions if the partial pressure of nitrogen, carbon dioxide, and other gases are 79.1 KPa, 0.04 KPa, and 0.94 KPa respectively? • Ptotal = PO + PN + PCO + POther gases 2 2 2 • 101.3 KPa = PO2 + 79.1 KPa + 0.04 KPa + 0.94KPa • PO = 101.3 KPa – (79.1 KPa + 0.04 KPa + 0.94KPa) 2 • PO = 21.2 KPa 2 Two 1.0 L containers, A and B, contain gases with 2.0 atm and 4.0 atm, respectively. Both gases are forced into Container Z (vol. 2.0 L). Find the total pressure of mixture in Z. A B Z Two 1.0 L containers, A and B, contain gases with 2.0 atm and 4.0 atm, respectively. Both gases are forced into Container Z (vol. 2.0 L). Find the total pressure of mixture in Z. A A B Z PX VX 2.0 atm 1.0 L VZ PX,Z 1.0 atm 2.0 L B 4.0 atm 1.0 L 2.0 atm Total = 3.0 atm Find total pressure of the gas mixture in Container Z. A 1.3 L 3.2 atm B 2.6 L 1.4 atm C Z 3.8 L 2.7 atm 2.3 L X atm Find total pressure of the gas mixture in Container Z. A B 1.3 L 3.2 atm 2.6 L 1.4 atm PX VX A 3.2 atm 1.3 L B 1.4 atm 2.6 L C 2.7 atm 3.8 L C Z 3.8 L 2.7 atm 2.3 L X atm VZ PX,Z 1.8 atm 2.3 L 1.6 atm 4.5 atm Total = 7.9 atm Dalton’s Law Zumdahl, Zumdahl, DeCoste, World of Chemistry 2002, page 422 Dalton’s Partial Pressures Zumdahl, Zumdahl, DeCoste, World of Chemistry 2002, page 421 Dalton’s Law of Partial Pressures The mole ratio in a mixture of gases determines each gas’s partial pressure. Total pressure of mixture (3.0 mol He and 4.0 mol Ne) is 97.4 kPa. Find partial pressure of each gas Dalton’s Law of Partial Pressures Total pressure of mixture (3.0 mol He and 4.0 mol Ne) is 97.4 kPa. Find partial pressure of each gas. PHe 3 mol He 97.4 kPa 7 mol gas 41.7 kPa PNe 4 mol Ne 97.4 kPa 7 mol gas 55.7 kPa 80.0 g each of He, Ne, and Ar are in a container. The total pressure is 780 mm Hg. Find each gas’s partial pressure. 80.0 g each of He, Ne, and Ar are in a container. The total pressure is 780 mm Hg. Find each gas’s partial pressure. 1mol 80 g He 20 mol He 4g 1mol 80 g Ne 4 mol Ne 20 g 1 mol 80 g Ar 2 mol Ar 40 g PHe = 20/26 of total Total: 26 mol gas PNe = 4/26 of total PAr = 2/26 of total PHe 600 mm Hg, PNe 120 mm Hg, PAr 60 mm Hg Example: A student generates oxygen gas and collects it over water. If the volume of the gas is 245 mL and the barometric pressure is 758.0 torr at 25oC, what is the volume of the “dry” oxygen gas at STP? (Pwater = 23.8 torr at 25oC) PO2 = PT - Pwater = 758.0 torr - 23.8 torr = 734.2 torr Find the molar mass of an unknown gas if a 0.16 g sample of the gas is collected over water and equalized to a pressure of 781.7 torr and a volume of 90.0 mL at a temperature of 28°C . Find the molar mass of an unknown gas if a 0.16 g sample of the gas is collected over water and equalized to a pressure of 781.7 torr and a volume of 90.0 mL at a temperature of 28°C . 44 g/mol Homework • Do the AP sample problem (1999 Test question #5) in notebook. It will be included as part of your homework. • Don’t forget the pre-lab and lab summary for “The Molecular Mass of a Volatile Liquid”. Gas Diffusion and Effusion Graham's Law: governs the rate of effusion and diffusion of gas molecules. “Stink” or “Die” a The Root Mean Square Speed Fig. 10.17 Page 285 To use Graham’s Law, both gases must be at same temperature. diffusion: particle movement from high to low concentration NET MOVEMENT effusion: diffusion of gas particles through an opening For gases, rates of diffusion & effusion obey Graham’s law: more massive = slow; less massive = fast Gas Diffusion and Effusion Graham's Law: governs the rate of effusion and diffusion of gas molecules. Rate of A molar mass of B Rate of B molar mass of A Rate of diffusion/effusion is inversely proportional to its molar mass. 35 36 Br Graham’s Law 79.904 Kr 83.80 Determine the relative rate of diffusion for krypton and bromine. The lightest gas is “Gas A” and the heavier gas is “Gas B”. Relative rate means find the ratio “vA/vB”. vA vB v Kr v Br2 m Br2 m Kr mB mA 159.80g/mol 1.381 83.80g/mol Kr diffuses 1.381 times faster than Br2. 1 8 H O Graham’s Law 1.00794 15.9994 A molecule of oxygen gas has an average speed of 12.3 m/s at a given temp and pressure. What is the average speed of hydrogen molecules at the same conditions? vA vB mB mA vH 2 12.3 m/s 32.00 g/mol 2.02 g/mol vH 2 vH 2 vO2 mO2 mH 2 12.3 m/s 3.980 vH2 49.0m/s 1 8 H2 Graham’s Law 2.0 O 15.9994 An unknown gas diffuses 4.0 times faster than O2. Find its molar mass. The lightest gas is “Gas A” and the heavier gas is “Gas B”. The ratio “vA/vB” is 4.0. vA vB vA v O2 mB mA mO2 mA Square both sides to get rid of the square root sign. 32.00 g/mol g/mol 32.00 4.0 m A A 32.00 g/mol 16 mA 32.00 g/mol mA 2.0 g/mol 16 2 Kinetic Molecular Theory Theory developed to explain gas behavior. • Theory of moving molecules. • Assumptions: – Gases consist of a large number of molecules in constant random motion. – Volume of individual molecules negligible compared to volume of container. – Intermolecular forces (forces between gas molecules) negligible. – Energy can be transferred between molecules, but total kinetic energy is constant at constant temperature. – Average kinetic energy of molecules is proportional to temperature. Kinetic Molecular Theory Kinetic molecular theory gives us an understanding of pressure and temperature on the molecular level. • Pressure of a gas results from the number of collisions per unit time on the walls of container. • Magnitude of pressure given by how often and how hard the molecules strike. • Gas molecules have an average kinetic energy. • Each molecule has a different energy. Kinetic Molecular Theory There is a spread of individual energies of gas molecules in any sample of gas. As the temperature increases, the average kinetic energy of the gas molecules increases Kinetic Molecular Theory As kinetic energy increases, the velocity of the gas molecules increases. • Root mean square speed, u, is the speed of a gas molecules having the certain average kinetic energy. • Average kinetic energy, , is related to root mean square speed, u: 2 1 2 mu a Kinetic Molecular Theory As kinetic energy increases, the velocity of the gas molecules increases. • Root mean square speed, u, is the speed of a gas molecules having the certain average kinetic energy. • Average kinetic energy, , is related to root mean square speed, u: 2 1 2 mu a How does this theory explain Boyles Law? As the volume of a container of gas increases at constant temperature, the gas molecules have to travel further to hit the walls of the container. There are fewer collisions by the gas molecules with the walls of the container. Therefore, pressure decreases. If temperature increases at constant volume, the average kinetic energy of the gas molecules increases. Therefore, there are more collisions with the container walls and the pressure increases. How does this theory explain Charles Law? If temperature increases at constant volume, the average kinetic energy of the gas molecules increases and they speed up. Therefore, there are more frequent and more forceful collisions with the container walls by the gas molecules and the pressure increases. Ideal Gases vs. Real Gases • An ideal gas is an “imaginary gas” made up of particles with negligible particle volume and negligible attractive forces. Ideal Gases vs. Real Gases • In a “Real Gas” the molecules of a gas do have volume and the molecules do attract each other. • Therefore anything that makes gas particles more likely to stick together or stay close to one another make them behave less ideally. Real Gases: Deviations from Ideal Behavior As the pressure on a gas increases, the molecules are forced into a smaller volume. • As the volume becomes smaller, the molecules get closer together, and a greater fraction of the occupied space is actually taken up by gas molecules. • Therefore, the higher the pressure, the less the gas resembles an ideal gas. Real Gases: Deviations from Ideal Behavior • The smaller the distance between gas molecules, the more likely attractive forces will develop between the molecules. • As temperature increases, the gas molecules move faster and are further apart. • Also, higher temperatures mean more energy available to break intermolecular forces. • Therefore, the higher the temperature, the more ideal the gas. Real Gases and Ideal Behavior • A real gas typically exhibits behavior closest to “ideal gas” behavior at low pressures and high temperatures. Real Gases: The van der Waals equation We add two terms to the ideal gas equation one to correct for volume of molecules and the other to correct for intermolecular attractions The correction terms generate the van der Waals equation: nRT n 2 a P 2 V nb V where a and b are empirical constants. 2 n P a V nb nRT 2 V a corrects for the effect of molecular attractions (van der Waals forces), and b corrects for the molecular volume Real Gases: The van der Waals equation We add two terms to the ideal gas equation one to correct for volume of molecules and the other to correct for intermolecular attractions The correction terms generate the van der Waals equation: • You will not be required to solve this equation but you should know its form and which variables need to be corrected. 2 n P a V nb nRT 2 V a corrects for the effect of molecular attractions (van der Waals forces), and b corrects for the molecular volume