Gas Laws
advertisement

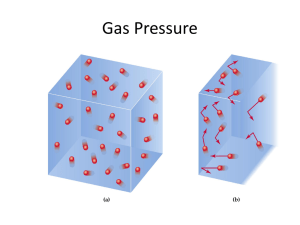
Gas Laws The Gas Laws • Describe HOW gases behave. • Can be predicted by the The Kinetic Theory Kinetic Theory states that all matter consists of tiny particles that are in constant motion Matter Review • Solids: least motion, definite volume definite shape • Liquids: more motion, particles further apart than solids (can pour), definite volume but indefinite shape • Gases: most motion of three states, indefinite volume and indefinite shape 4 things • In order to completely describe a gas you need to measure 4 things 1. 2. 3. 4. Pressure Temperature Volume Number of particles Gas Pressure • Pressure is defined as force per unit area. • Gas particles exert pressure when they collide with the walls of their container. • The SI unit of pressure is the pascal (Pa). • However, there are several units of pressure – Pascal (Pa) – Kilopascal (KPa) – Atmosphere (atm) 1 atm 4 Liters • As the pressure on a gas increases 2 atm 2 Liters • …the volume decreases • Pressure and volume are inversely related Temperature • Raising the temperature of a gas increases the pressure if the volume is held constant. • The molecules hit the walls harder. • The only way to increase the temperature at constant pressure is to increase the volume. 300 K • If you start with 1 liter of gas at 1 atm pressure and 300 K • and heat it to 600 K one of 2 things happens 600 K 300 K • Either the volume will increase to 2 liters at 1 atm 300 K •Or the pressure will increase to 2 atm. •More collisions mean greater pressure 600 K Changing the Size of the Container • In a smaller container molecules have less room to move • Hit the sides of the container more often • As volume decreases pressure increases. The effect of adding gas • When we blow up a balloon we are adding gas molecules. • Doubling the number of gas particles doubles the pressure More molecules means more collisions Fewer molecules means fewer collisions. • If you double the number of molecules… 1 atm • …You double the pressure 2 atm 4 atm • As you remove molecules from a container…….. 2 atm • ….the pressure decreases 2 Avogadro’s Hypothesis Nitrogen, N2 Hydrogen, H2 Oxygen, O2 1 mole of each gas has the same number of molecules at STP Boyle’s Law • At a constant temperature, pressure and volume are inversely related • As one goes up the other goes down • P1 x V1= P2 x V2 Example • A balloon is filled with 25 L of air at 1.0 atm pressure. If the pressure is changed to 1.5 atm what is the new volume? P1= 1 atm V1= 25 L P2= 1.5 atm V2= ? (1 atm)(25 L)=(1.5 atm)(V2) 16.7 L Example • A balloon is filled with 73 L of air at 1.3 atm pressure. What pressure is needed to change to volume to 43 L? • A sample of Helium gas is compressed from 4.0 L to 2.5 L at a constant temperature. If the pressure of the gas in the 4.0 L volume is 210 kPa, what will the pressure be at 2.5 L? Charles’s Law • The volume of a gas is directly proportional to the Kelvin temperature when the pressure is held constant Example • A sample of gas at 40.0 °C occupies a volume of 2.32 L. If the temperature is raised to 75.0 °C what will the new volume be? V1= 2.32 L T1= 40+273 V2= ? T2= 75+273 V1 = V2 T1 T2 2.32 L = V2 313K 348 K MUST CONVERT TEMP TO K!!!!!!! 2.58 L Examples • What is the pressure inside a 0.250 L can of deodorant that starts at 25ºC and 1.2 atm if the temperature is raised to 100ºC? • At what temperature will the can above have a pressure of 2.2 atm? Combined Gas Law • The Combined Gas Law Deals with the situation where only the number of molecules stays constant. • P1 x V1 = P2 x V2 T1 T2 Example • A gas at 110.0 kPa and 30.0°C fills a flexible container to a volume of 2.00 L. If the temperature was raised to 80.0°C and the pressure was increased to 440.0 kPa, what is the new volume? Example • P1V1 = P2V2 T1 T2 • • • • • • P1 = 110.0 kPa V1 = 2.00 L T1 = 30.0 °C = 303 K P2 = 440.0 kPa V2 = ? T2 = 80.0 °C = 353 K Example • P1V1 = P2V2 T1 T2 • (110.0)(2.00L) = (440.0kPa)(V2) 303K 353K • V2 = 0.583 L Dalton’s Law of Partial Pressures • The total pressure inside a container is equal to the sum of the partial pressure due to each gas. • The partial pressure of a gas is the contribution by that gas hitting the wall. • PTotal = P1 + P2 + P3 + … • We can find out the pressure in the fourth container • By adding up the pressure in the first 3 2 atm 1 atm 3 atm 6 atm Dalton’s Law of Partial Pressures A gas mixture contains H2, He, Ne, and Ar. The total pressure of the mixture is 93.6 kPa. The partial pressures of He, Ne, and Ar are 15.4 kPa, 25.7 kPa, and 35.6 kPa respectively. What is the pressure exerted by H2? PT = PH2 + PHe + PNe + PAr PH2 = 16.9 kPa Dalton’s Law of Partial Pressures A person using an oxygen mask is breathing air with 33% Oxygen. What is the partial pressure of the Oxygen when the air pressure in the mask is 110 kPa? 33% of 110 kPa 36 kPa Diffusion & Effusion Molecules moving from areas of high concentration to low concentration. Perfume molecules spreading across the room. Effusion - Gas escaping through a tiny hole in a container. Both depend on the speed of the molecules Diffusion • Bigger molecules move slower at the same temp. • Bigger molecules effuse and diffuse slower • Helium effuses and diffuses faster than air -escapes from balloon. Kinetic Molecular Theory Three main points to the kinetic theory of gases. • Gases are made of small particles, which are spread very far apart from each other and behave independently of one another. • Gas particles constantly move, randomly, yet in a straight line until acted upon by an outside force or barrier. • All collisions are perfectly elastic which means that no energy is gained or lost during the collision. Ideal Gases • We are going to assume that gases behave ideally • Does not really exist • Assume particles have no volume • Assume no attractive forces between molecules ONLY 2 ELEMENTS TO BEHAVE MOST LIKE AN IDEAL GAS ARE HYDROGEN AND HELIUM Ideal Gases vs Real Gases Real Gases deviate from the Ideal Gases: 1) Volume of a gas is significant (22.4 L) 2) Gas particles can condense, so do have forces of attraction between particles Real gases differ when at low temp and high pressure