Early stage Hodgkin lymphoma – is it no longer a problem
advertisement

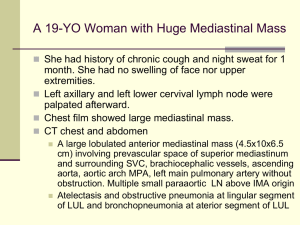
Early Stage Hodgkin Lymphoma: Latest Concepts & Controversies Morton Coleman, M.D. Director, Center for Lymphoma and Myeloma Weill Cornell Medical Center New York Presbyterian Hospital New York, New York THE MAIN INTENT: LESS TOXICITY At least 85% of Hodgkin patients can anticipate a cure. The charge: cure even more patients with the least impact on their well being EARLY STAGE HODGKIN HISTORY • 5-10 yr. relapse rate lower for chemotherapy + IF RT than EF RT alone (JCO 24: 3128-35, 2006; JC0 25: 3495-3502, 2007; NEJM 357: 1916-27, 2008) • No difference in OS at 4-5-yrs. between ABVD alone and ABVD + RT for patients with limited stage non-bulky HL (Blood 104: 3483-89, 2004; JCO 23: 4634-42, 2005) • RT is associated with late 2nd malignancies and cardiovascular events especially after 10 yrs. (JCO 21: 3431-9, 2003; NEJM 355: 1572-82, 2006; JCO 27 [Suppl.] abs. 8547, 2009 courtesy Straus,D Kaplan–Meier Estimates of Overall Survival and Freedom from Disease Progression. Meyer RM et al. N Engl J Med 2012;366:399-408 Kaplan–Meier Estimates of Overall Survival and Freedom from Disease Progression among Patients with an Unfavorable Risk Profile. Meyer RM et al. N Engl J Med 2012;366:399-408 What role does PET Scans play in this effort? May interim PET/CAT scans be of value or should scans be used only at the end of treatment? In Vivo Treatment Sensitivity With Positron Emission /Computed Tomography After One Cycle of Chemotherapy for Hodgkin Lymphoma Martin Hutchings, Lale Kostakoglu, Morton Coleman, et al. JCO 32: 2705-2711, 2014 FDG-PET: After one (two treatments) versus two cycles (four treatments) of therapy Early determination of treatment sensitivity in Hodgkin lymphoma: FDG-PET/CT after one cycle of therapy has a higher negative predictive value than after two cycles of therapy Participating Nations Denmark United States Italy Poland (Nine Institutions) Patient Population:126 Pts. • Stage I 8% • Stage 2 46% • Stage 3 19% • Stage4 27% • B Sxs 56% • Bulky 37% Comparison of the prognostic value of PET 1 and PET 2: Progression Free Survival at 2 Years PET 1 PET2 • Negative predictive value 98% 91% • Positive predictive value 63% 85% • Sensitivity 94% 61% • Specificity 86% 97% • Concordance >90% Involved Field Radiotherapy Versus No Further Treatment in Patients with Early- Stage Hodgkin Lymphoma and Negative PET Scan After 3 ABVD Cycles: Resuts of the UK NCRI RAPID Trial Proc ASH 120,2012; Abstract 547 Radford J, Barrington S, Counsell N, et al RAPID Trial Design Initial treatment: ABVD x 3 Reassessment: 571pts if NR/PD, patient goes off study if CR/PR, FDG-PET scan performed PET-positive (145pts) 4th cycle ABVD then IFRT Radford J, et al. Blood. 2012;120: Abstract 547. PET negative (420 pts) Randomization IFRT (209 pts) No further treatment (211pts) Outcomes After Median Follow-Up of 45.7 Months PET negative; randomized to IFRT (n = 209) PET negative; randomized to NFT (n = 211) PET positive; 4th cycle ABVD/IFRT (n = 145) Progressions 9 20 11 Deaths 8 1 8 PFS at 3 years 93.8% 90.7% 85.9% OS at 3 years 97.0% 99.5% 93.9% Radford J, et al. Blood. 2012;120: Abstract 547. Summary • 602 pts registered between 2003 and 2010 • 75% PET-negative at central review after ABVD x 3 • In the randomized PET-negative population, 3 yr PFS is 92.8% IFRT and 90% NFT • Risk difference -3% is within the maximum allowable difference of -7% Radford J, et al. Blood. 2012;120: Abstract 547. Commentary • These data are similar to those reported from Argentina several years ago for all stages of disease when PETs were obtained after 3 cycles (Pavlosky, et al.) • CAUTION: Were the deaths in the IFRT arm ‘flukes’ and not related to the IFRT? If so, would the data and conclusions be different. Radford J, et al. Blood. 2012;120: Abstract 547. An Individual Patient-Data Comparison of Combined Modality Therapy and ABVD Alone for Patients with Limited Stage Hodgkin Lymphoma A study of the NCIC (HD 6) and the German Hodgkin Study Group (HD 10 &11) Hay AE, Klimm B, Chen BE, et al Annals of Oncology 24 (12) 3065-3069, 2013 GHSG Early-Stage HL Risk Factors Favorable: CS IA,IB, IIA, IIB without risk factors Unfavorable: CS IA, IB, with at least one of the risk factors a-d given below or CS IIB with risk factor c, d, or both given below: and IIA a) Large mediastinal mass (≥1/3 of maximum transverse thorax diameter) b) Extranodal involvement c) High erythrocyte sedimentation rate (≥50 mm/h in patients without B-symptoms, ≥30 mm/h in patients with Bsymptoms) d) 3 or more involved lymph node areas Eich HT, et al. J Clin Oncol. 2011;28:4199-4206. HD.6 Trial Patients with Clinical Stage I-IIA Hodgkin Lymphoma Study schema of a randomized trial comparing a strategy that includes radiation therapy with ABVD in patients with limited-stage Hodgkin lymphoma Exclude low-risk patients Exclude high-risk patients Stage IA with single node of Hodgkin lymphoma and all of: • Lymphocyte predominant or nodular sclerosis histology • Bulk <3cm • ESR <50 mm/hour • Disease involving high neck or epitrochlear region only Patients with either: • Bulk >10 cm or ≥1/3 chest wall diameter, or • Intra-abdominal disease Favorable or unfavorable cohort Stratify Randomly Assign Unfavorable cohort patients have any of: • Age ≥40 years • ESR ≥50 mm/hour • Mixed cellularity or lymphocyte deplete histology • ≥4 sites of disease Treatment that includes radiation therapy • Favorable cohort: subtotal nodal radiation therapy • Unfavorable cohort: combined modality therapy with ABVD x 2 cycles plus subtotal nodal radiation therapy Meyer RM, et al. N Engl J Med. 2012;366:399-408. ABVD as a single modality • Both cohorts: ABVD x 2 cycles • IF CR or CRu, ABVD x 2 more cycles (total 4 cycles) • If <CR or CRu, ABVD x 4 more cycles (total 6 cycles) Comparison of NCIC CTG HD.6 and GHSG HD10 and HD11 Staging, Eligibility and Preferred Arms 2 ABVD + 20 Gy IFRT Early, favorable HD10 4 ABVD + 30 Gy IFRT Early, unfavorable HD11 Advanced GHSG 4 – 6 ABVD alone NCIC CTG HD.6 Favorable Unfavorable Not necessarily to scale Hay AE, et al. Blood. 2012;120: Abstract 549. Advanced Comparison of NCIC CTG HD.6 and GHSG HD10 and HD11 Staging, Eligibility and Preferred Arms Very good prognosis Early, favorable HD10 B or Bulk Early, unfavorable HD11 Advanced GHSG NCIC CTG HD.6 Favorable Unfavorable Advanced Not necessarily to scale Hay AE, et al. Blood. 2012;120: Abstract 549. Attribution of Death: All Patients Cause of Death Number Med. F/U GHSG HD10/11 406 7.6 Years NCIG CTG HD.6 182 11.2 Years Hodgkin lymphoma Immediate toxicity 5 2 4 1 Second cancer Cardiac Other 2 4 6* 3 2 0 Total 19 10 *Other deaths were: 1 suicide, 1 respiratory failure, 1 cerebral hemorrhage, 1 progression of NHL, 2 unknown Hay AE, et al. Blood. 2012;120: Abstract 549. Outcomes: All Patients GHSG HD10/11 NCIG CTG HD.6 406 7.6 Years 182 11.2 Years 8-yr TTP 93% 8-yr PFS 8-yr OS Endpoint Number Med. F/U HR (95% CI) GHSG PD/OS NCIC CTG PD/OS 87% 0.44 (0.24, 0.78) 25/0 23/0 89% 86% 0.71 (0.42, 1.18) 25/13 23/4 95% 95% 1.09 (0.49, 2.40) 19 10 Hay AE, et al. Blood. 2012;120: Abstract 549. Overall Commentary • Combined modality therapy (CMT) improves disease control by 4%-7% • Could this difference in control have been obviated by the use of PET scans after one cycle of therapy since there was a 7% difference in PET negative results between cycles 1 and 2, much less cycle 3? • The relatively long term outcomes associated with IFRT remain to be clarified Omitting Radiotherapy in Early Positron Emission TomographyNegative Stage I/II Hodgkin Lymphoma Is Associated With an Increased Risk of Early Relapse: Clinical Results of the Preplanned Interim Analysis of the Randomized EORTC/LTSA/FIL H10 Trial • Raemaekers, J.M.M., Andre, Marc P.E., Federico, M. JCO 32:1188-1194, 2014 Study design of European Organisation for Research and Treatment of Cancer, Lymphoma Study Association, and Fondazione Italiana Linfomi H10 20551 trial of patients age 15 to 70 years with untreated supradiaphragmatic clinical stage I/II Hodgkin lymphoma Raemaekers J M et al. JCO 2014;32:1188-1194 2014 by American Society of Clinical Oncology Flowchart of patients included in interim analysis. Raemaekers J M et al. JCO 2014;32:1188-1194 2014 by American Society of Clinical Oncology Results: Progression Favorable: no RT – 9 (5%) with RT-1 (0.5%) Unfavorable: no RT-16 (5%) with RT-7 (2.6%) Study is now closed. Abstract No: CT-08 Early-Stage Hodgkin's Disease: The Utilization of Radiation Therapy and Its Impact on Overall Survival Rahul R. Parikh, M.D.1, Joachim Yahalom, M.D.2, James A. Talcott, M.D.3, Michael L. Grossbard, M.D.3, & Louis B. Harrison, M.D.4 Mt. Sinai Beth Israel Medical Center & Mt. Sinai St. Luke’s-Roosevelt Hospitals, Mount Sinai Health System, Department of Radiation Oncology, New York, NY 2 Memorial Sloan-Kettering Cancer Center, Department of Radiation Oncology, New York, NY 3 Mt. Sinai Beth Israel Medical Center & Mt. Sinai St. Luke’s-Roosevelt Hospitals, Mount Sinai Health System, Department of Hematology-Oncology, New York, NY 4 Department of Radiation Oncology, Moffitt Cancer Center and Research Institute, Tampa, FL 1 Background National Cancer Database (NCDB) Joint program of the Commission on Cancer and the American Cancer Society Prospectively collected; Nationwide outcomes database covering 75% of all newly diagnosed cancers (>1,500 U.S. hospitals); Not population-based dataset Not geographically limited (care in all states included) Reflects contemporary treatment programs RT specifics available for analysis (modality, dose, fx, volume/site) Aims of the Study Primary Endpoint: Determine relationship between use of RT and overall survival in patients with earlystage Hodgkin’s Disease Secondary Endpoints: Determine the trends of utilization rates of RT Determine other factors (socioeconomic factors, timing of chemotherapy, co-morbid conditions) associated with overall survival Methods Hodgkin’s Disease, Stage I/II, diagnosed 1998-2011 (N = 41,420) Median f/u = 6.4 years; Median age = 37 years (range: 18-90) Multi-agent chemotherapy given to 96% of the pts Did NOT receive Radiation Therapy Received Radiation Therapy as part of CMT (N = 20,897, 51%) (N = 20,523, 49%) Median RT dose = 30.6 Gy Evaluated clinical features & survival outcomes The association between RT use, co-variables, and outcome was assessed in a multivariate Cox proportional hazards model. Survival was estimated using the Kaplan-Meier method. Univariate Survival Analysis (OS) Prognostic Factor HR (95% CI) p-value RT <0.0001* No 1.00 (Ref.) Yes 0.51 (0.48-0.54) Age (years) at Diagnosis ≤ 40 > 40 Gender Male Female Stage 1 2 Presence of “B” Symptoms No Yes Charlson/Deyo comorbidity score 0 1 2 Transplant Proc. No Yes <0.0001* 0.19 (0.180.20) 1.00 (Ref.) <0.0001* 1.00 (Ref.) 0.81 (0.76-0.86) <0.0001* 1.00 (Ref.) 0.70 (0.66-0.74) <0.0001* 1.00 (Ref.) 1.65 (1.46-1.87) <0.0001* 1.00 (Ref.) 3.14 (2.76-3.57) 5.19 (4.23-6.36) 1.00 (Ref.) 1.64 (1.15-2.35) 0.012* Prognostic Factor Timing of Chemotherapy ≤30 days from dx >30 days from dx Education (% not HS grad) ≥ 29% 20-28.9% 14-19.9% <14% Household Income < $30,000 $30,000-34,999 $35,000-45,999 ≥ $46,000 Insured Status Insured (Medicare, Private / Managed Care) Not Insured / Medicaid Facility Type Comm. Cancer Prog. Comprehen Cancer Prog Academic/Res. Prog. Other HR (95% CI) p-value <0.0001* 0.67 (0.640.72) 1.00 (Ref) <0.0001* 1.00 (Ref.) 0.84 (0.76-0.92) 0.75 (0.69-0.82) 0.62 (0.56-0.67 1.00 (Ref.) 0.92 (0.83-1.01) 0.79 (0.72-0.87) 0.62 (0.57-0.68) <0.0001* <0.0001* 1.00 (Ref.) 5.27 (4.97-5.59) <0.0001* 1.00 (Ref.) 0.84 (0.77-9.23) 0.66 (0.60-0.73) 0.96 (0.77-1.19) Factors Associated with Overall Survival (MVA) HR LCL UCL Utilization of RT (yes vs. no) 0.47 0.43 0.53 Age (≤40 vs. >40) 0.23 0.20 0.26 Race (black vs. white) 0.93 0.79 1.11 Race (other vs. white) 0.81 0.69 0.91 Insured status (Uninsured vs. Insured) 3.48 3.13 3.87 Stage (1 vs. 2) 0.89 0.77 1.02 B Symptoms (yes vs. no) 1.72 1.50 1.97 Transplant performed (yes vs. no) 2.66 1.59 4.45 Co-morbidity score (1 vs. 0) 1.94 1.69 2.23 Co-morbidity score (2 vs. 0) 2.93 2.34 3.65 Timing of chemo (≤30 days vs. >30days) 0.84 0.76 0.94 0.1 1.0 Inc. Overall Survival 10.0 Dec. Overall Survival Overall Survival by RT use 84% 76% RT Utilization 90 56% 80 1998 70 41% 2011 ` Percent of patients receiving RT 100 60 50 40 30 20 10 0 1998 2000 2002 2004 2006 Year of Diagnosis 2008 2010 Reason for No Radiation Freq. % Radiation was not part of the planned initial treatment strategy 18,482 86.30 Radiation was contraindicated Radiation recommended but not administered Radiation recommended but refused by the patient Radiation recommended, unknown whether delivered Unknown if recommended or administered Total 185 1,041 279 872 561 21,420 0.86 4.86 1.30 4.07 2.62 100.00 Conclusions Largest contemporary dataset of patients with earlystage HD (n=41,420) The use of RT is associated with improved 10-yr OS (84% vs. 76%, HR=0.51, p<0.00001) BUT THIS MAY BE DUE TO A POPULATION WITH AN INHERENT BETTER PROGNOSIS Utilization of RT has decreased by 15% from 1998 to 2011 (5641%; not part of initial treatment strategy) Specific factors (socioeconomic, insurance status, facility type) were associated with underutilization of RT which may be targeted to improve patient access to RT, IF RT IS INDEED INDICATED Brentuximab Vedotin Mechanism of Action Brentuximab vedotin (SGN-35) ADC monomethyl auristatin E (MMAE), potent antitubulin agent protease-cleavable linker anti-CD30 monoclonal antibody ADC binds to CD30 ADC-CD30 complex traffics to lysosome MMAE is released MMAE disrupts microtubule network G2/M cell cycle arrest Apoptosis Frontline Therapy With Brentuximab Vedotin Combined with ABVD or AVD in Patients with Newly Diagnosed Advanced-Stage Hodgkin Lymphoma Abstract 798, ASH 2012 Ansell SM, Connors JM, Park SI, et al Study Design • Phase I, multicenter, dose-escalation study • Major eligibility criteria – Treatment-naïve HL patients – Age ≥18 to ≤60 years – Stage IIA bulky disease or Stage IIB-IV disease • Treatment design – 28-day cycles (up to 6 cycles) with dosing on Days 1 and 15 – Dose escalation cohorts – I-6, II-13, III-6, IV-6, expansion-20 Cycle 1 Cycle 3 Cycle 2 Brentuximab Vedotin A(B)VD 6 Cycles +/- XRT 0 2 4 6 Weeks Ansell SM, et al. Blood. 2012;120: Abstract 798. 8 10 12 Response Results at End of Front-Line Therapy ABVD with brentuximab vedotin N = 22 AVD with brentuximab vedotin N = 25 Complete remission 21 (95) 24 (96) Progressive disease 0 1 (4) 1b (5) 0 Response per Investigatora Response at end of front-line therapy, n (%) Not evaluable due to AEs a Assessed using Cheson 2007 b Patient had a Grade 5 event of pulmonary toxicity prior to the end of front-line therapy • • Response results at end of front-line therapy: ◦ ABVD cohorts: 21 of 22 CR (95%) ◦ AVD cohorts: 24 of 25 CR (96%) In addition, 1 patient withdrew consent and 3 patients were lost to follow-up prior to completion of front-line therapy and were not evaluable for response Ansell SM, et al. Blood. 2012;120: Abstract 798. CONCLUSIONS: EARLY STAGE HL PET SCANS HAVE ALLOWED THE REDUCTION OF CHEMOTHERAPY CYCLES IN EARLY STAGE HL. COMBINED MODALITY THERAPY (CMT)PROVIDES ABOUT ABOUT A 5% (+/- 3%)ADVANTAGE OVER CHEMOTHEAPY ALONE IN PFS ALTHOUGH OS ADVANTAGE REMAINS TO BE PROVEN. THE LONG TERM TOXICITY OF LIMITED OR NODAL FIELD RT IS STILL UNKNOWN. ACUTE TOXICITY MAY PLAY A ROLE IN REDUCING OS? PET SCANS AFTER 1 CYCLE MAY REDUCE THE PFS ADVANTAGE OF CMT. PATIENT SELECTION IS CRITICAL IN DECIDING WHAT RX TO ADMINISTER. WILL BRENTUXIMAB VEDOTIN REPLACE THE ‘NEED’ FOR RT? Acknowledgment Clinical Research (Cornell) Jia Ruan, M.D., Ph.D. Richard Furman, M.D. John P. Leonard, M.D. Peter Martin, M.D. Maureen Joyce, R.N. Patricia Glenn, R.N. Jamie Ketas Jessica Hansen Karen Weil Jennifer O’Loughlin Laboratory Research Ari Milneck, M.D., Ph.D.(Cornell) Katherine Hajjar, M.D. (Cornell) Shahin Rafii, M.D. (Cornell) Translational Core Maureen Lane, Ph.D. (Cornell) Maureen Ward Biostatistician Madhu Mazumdar, Ph.D. (Cornell) Lymphoma Research Foundation ASCO Foundation (YIA, CDA) NIH / NHLBI THANK YOU FOR YOUR ATTENTION