- Oncology Exchange
advertisement

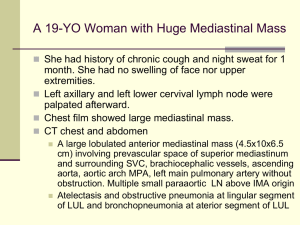
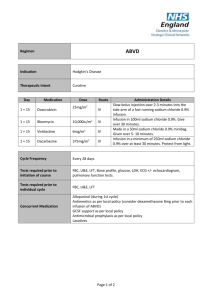
A CME-certified Oncology Exchange Program Jointly provided by Potomac Center for Medical Education and Rockpointe Supported by an educational grant from Seattle Genetics, Inc. Faculty Anas Younes, M.D. Chief, Lymphoma Service Memorial Sloan Kettering Cancer Center New York Jeremy S. Abramson, M.D. Director, Center for Lymphoma Massachusetts General Hospital Cancer Center Off-label Discussion Disclosure This educational activity may contain discussion of published and/or investigational uses of agents that are not indicated by the Food and Drug Administration. PCME does not recommend the use of any agent outside of the labeled indications. Please refer to the official prescribing information for each product for discussion of approved indications, contraindications and warnings. The opinions expressed are those of the presenters and are not to be construed as those of the publisher or grantors. Learning Objectives • Assess emerging frontline approaches for patients with Hodgkin lymphoma • Evaluate the efficacy and safety data for novel agents in patients with relapsed/refractory Hodgkin lymphoma • Discuss strategies to mitigate adverse effects to improve long-term survival in Hodgkin lymphoma Hodgkin Lymphoma Introduction and Initial Therapy Case Study 1 • M.C. is a 23 yo woman who presents with painless swelling of a right supraclavicular lymph node. She was otherwise asymptomatic with a normal physical examination • A course of antibiotics was prescribed, without response • An FNA was performed and interpreted as consistent wit a reactive lymphoid population • An excisional lymph node biopsy was ultimately performed CD15 CD30 CD3 EBER Staging and Pre-treatment Evaluation • PET/CT showed non-bulky stage IIA disease • CBC, CMP unremarkable • ESR 12 ml/hour • HIV negative • No bone marrow is performed • Echocardiogram and PFTs show no organ dysfunction • What is the optimal therapy for this patient? Case 1: How will you treat this patient? 1. ABVD x 6 cycles followed by 30 Gy radiation 2. ABVD x 4 cycles followed by 30 Gy radiation 3. ABVD x 2 cycles followed by 20 Gy radiation 4. ABVD x 4-6 cycles without radiation 5. Escalated BEACOPP +/- radiation Case 1: What if this patient was 73 years old instead of 23? 1. ABVD x 4 cycles followed by 30 Gy radiation 2. ABVD x 2 cycles followed by 20 Gy radiation 3. ABVD x 4-6 cycles without radiation 4. AVD x 4-6 cycles (omit bleomycin) +/- radiation 5. ChlVPP +/- radiation Epidemiology • ~9200 cases/year in US • ~1200 deaths • Median age 35 – Bimodal distribution • Slight male predominance • Incidence is stable Siegel, et al. CA Cancer J Clin. 2014; 64(1): 9-29. Clinical Presentation • 70% present with painless lymph node enlargement • Limited in 55%, Advanced in 45% • 30% will have “B” symptoms • Pruritus • Rare pain with alcohol ingestion • Sites of involvement – Nodal regions • • • • • • • • Cervical/Supraclavicular (L>R) 60-70% Mediastinal 60% Axillary 25-35% Hilar nodes 15-35% Para-aortic 30-40% Iliac 15-20% Inguinal 8-15% Mesenteric 1-4% – Other lymphoid organs Spleen 30-35% • Waldeyer’s ring 1-2% • – Extranodal sites (10-15%) Liver 2-6% • Bone marrow 2-8% • Other organs (lung, bone) 10% • Pathologic Classification • Classical HL – – – – • Nodular lymphocyte predominant HL Nodular sclerosis Mixed cellularity Lymphocyte rich Lymphocyte depleted CD20 NS LR MC LD Initial Evaluation and Workup • History • Preparation for chemotherapy • Physical exam – Echocardiogram • Staging studies – Pulmonary Function Tests, with DLCO – PET/CT scan – Bone marrow (Can usually be omitted based on PET findings) • Labs – CBC with differential – Erythrocyte Sedimentation Rate – Albumin, LFTs, Ca++, HIV – Fertility considerations Nodular Lymphocyte-predominant HL • 5% of Hodgkin lymphomas • Pathologically distinct from CHL • Clinically distinct from cHL – Indolent natural history – Male predominance (75%) – Median age in 30s – Increased risk among first degree family members – Limited stage in 75%, usually peripheral – Extranodal disease, B symptoms very uncommon – ~12% rate of high-grade transformation at 10y • Treatment – Radiation alone preferred for limited stage: 10 year FFP ~ 80% – Advanced stage extrapolated from cHL +/- rituximab • ABVD +/- rituximab • R-CHOP – Some suggest alkylators may be more effective (controversial) – Rituximab alone is an effective option, particularly at relapse – Late recurrences may occur Nodular LP vs Classical HL: GHSG Trials HD4-HD12 Freedom from Treatment Failure Overall Survival NLPHL NLPHL CHL Nogova, et al. JCO 2008 CHL Initial Treatment of Classical Hodgkin Lymphoma (cHL) Chemotherapy for cHL • MOPP developed at National Cancer Institute in 1964 – 54% freedom from progression at 10 years – Potentially sterilizing – Potentially leukemogenic • ABVD developed at Milan Cancer Institute in 1973 – Not sterilizing – Not associated with MDS or leukemia DeVita NEJM ‘03 MOPP versus ABVD for Advanced HL Failure-free Survival 5 year FFS MOPP-ABVD ABVD MOPP Canellos, et al. NEJM. 1992 65% 61% 50% Overall Survival 5 year OS MOPP-ABVD ABVD MOPP 75% 73% 66% Treatment Intensification: Escalated BEACOPP versus ABVD Viviani, et al. NEJM. 2011 Risk Factors in Advanced Stage CHL • Age ≥45 • Male Gender • Stage IV • Hemoglobin <10.5 g/dL • Leukocytosis >15k/mm3 • Lymphopenia <600/mm3 or 8% • Hypoalbuminemia <4.6 Moccia, et al. JCO 2012; 30(27): 3383-3388. 5y FFP (%) 5y OS (%) IPS N Original BCCA Original BCCA 0 57 84 88 89 98 1 195 77 84 90 97 2 195 67 80 81 91 3 155 60 74 78 88 4 88 51 67 61 85 ≥5 50 42 62 56 67 Advanced Stage disease • ABVD x 6 cycles • Outstanding questions – Selection of high-risk patients for treatment intensification – Role of PET-adapted therapy – Incorporation of novel agents Treatment of Limited Stage cHL Risk Stratification: Adverse factors in Limited Stage Disease EORTC GHSG • Large mediastinal mass • Large mediastinal mass • Age >50 • Extranodal disease • >4 involved sites • >3 nodal regions • ESR> 30 with B sx OR >50 without B sx • Elevated ESR Combined Modality Therapy is Superior to Radiation Alone in Early Favorable Disease Ferme et al. N Engl J Med 2007; 357: 1916-27. Combined Modality Therapy allows Reducing Chemotherapy Cycles and Radiation Field Ferme et al. N Engl J Med 2007; 357: 1916-27. Beware the Late Effects of Therapy Breast Cancer Incidence Ng et al. J Clin Oncol 2002, 20(8): 2101-2108; De Bruin et al. J Clin Oncol 2009 , 27(26): 4239-4246. Beware the Late Effects of Therapy Angina Pectoris Myocardial Infarction Aleman, et al. Blood 2007, 109(5): 1878-1886. All Cardiac Events German Hodgkin Study Group : HD10 trial CS I - II without risk factors* ABVD ABVD ABVD ABVD ABVD ABVD ABVD ABVD ABVD ABVD ABVD ABVD 30 Gy IF 20 Gy IF 30 Gy IF 20 Gy IF • No risk factors: Large mediastinal mass, extranodal disease, >3 nodal regions, elevated ESR Less is more: The GHSG HD10 trial Engert et al. N Engl J Med 2010; 363(7): 640-652. Chemotherapy alone for Limited-stage cHL Stage I/II HL (N = 399) Favorable (N = 123) 35Gy STNI ABVDx4-6 Unfavorable (N = 276) ABVDx2+STNI ABVDx4-6 • Study excluded patients with bulk – (M:T ratio >.33 or disease >10cm) • Unfavorable = ESR ≥ 50 mm/hr, age ≥ 40, > 3 sites, MC/LD Primary endpoint: 12 year overall survival Chemotherapy Alone Versus Radiation-containing Therapy at 12 Years OS at 12-years FFDP at 12-years • ABVD 94% • XRT/CMT 87% (HR 0.4; p=0.04) • ABVD 87% • XRT/CMT 92% (HR 3.03; p=0.01) Meyer, et al. N Engl J Med 2012; 366(5): 399-408. Are These Data Relevant in 2014? NO • Subtotal nodal radiotherapy is not the modern standard of care and leads to increased radiation exposure compared to current involved field/nodal techniques • XRT likely didn’t cause late infections, Alzheimer’s disease or drowning YES • Though we radiate lower volumes today, modern radiation in HL often includes the breasts, lungs, skin/soft tissues, and coronary ostia. • ABVD alone produced 12-year overall survival of 94%, which is excellent • This OS and FFDP is virtually identical to the GHSG HD11 trial results at 5 years using modern CMT with IFRT in the same population Can Interim PET Scans be Used to Direct use of Radiation Therapy? H10 F 2 ABVD 1 ABVD + INRT 30 Gy (+ 6 Gy) PET Arm events HR CI P Standard 1/188 1.00 Experimental 9/193 9.36 2.4535.73 .017 Arm events HR CI Standard 7/251 9.36 Experimental 16/268 2.42 R 2 ABVD H10 U 2 ABVD P E T + 2 ABVD 2 BEACOPPesc + INRT 30 Gy (+ 6 Gy) 2 ABVD + INRT 30 Gy (+ 6 Gy) PET P R 2 ABVD P E T - 4 ABVD + 2 BEACOPPesc + INRT 30 Gy (+ 6 Gy) Raemaekers, et al. J Clin Oncol, 2014, 32: 1-8. 1.35-4.36 .026 Who Should Receive Radiation? Not one size fits all • Current data support combined modality therapy for bulky limited stage disease • For non-bulky patients, choice of therapy should be informed by patient age, tumor location and patient preference • Further data anticipated regarding the role of PET-adapted therapy Treatment of Limited Stage disease • Favorable – ABVD x 2 cycles plus 20Gy IFRT – ABVD x 4-6 cycles without XRT (for selected patients) • Unfavorable, non-bulky – ABVD x 4 cycles + 30 Gy IFRT – ABVD x 6 cycles without XRT (for selected patients) • Unfavorable, bulky – ABVD x 6 cycles plus 30-36 Gy IFRT – ABVD x 4 cycles without XRT if interim PET negative (for selected patients) • Major ongoing questions – PET-adapted therapy – Incorporation of novel agents The Challenge of Treating Older Adults with Hodgkin Lymphoma Advanced Stage HL in Older Adults (E2496: ABVD/Stanford V) Overall survival Failure-free survival 90% 48% Probability Probability 74% P=0.002 58% P<0.0001 Year <60 yr ≥ 60 yr Year • 24% of older patients developed bleomycin lung toxicity • Treatment-related mortality: 9.3% vs. 0.3%, p<0.001) Evens et al. BJH, 2013, 161(1): 76-86. Limited Stage HL in Older Adults (GHSG HD10-HD11: ABVD x4 + IFRT) Overall Survival Progression-free Survival Time to HL death Time to HL treatment failure Boll et al. J Clin Oncol , 2013, 31(12): 1522-1529. Chicago Elderly HL Prognostic Model Adverse Risk factors: 1) ADL loss 2) >70 years Event-free survival 100 0 Prognostic factor(s) 1 Prognostic factor(s) 2 Prognostic factor(s) 75 Percent survival Percent survival 100 Overall survival 50 25 0 Prognostic factor(s) 1 Prognostic factor(s) 2 Prognostic factor(s) 75 50 25 0 0 0 30 60 90 120 Time in Months 150 180 0 30 60 90 Time in Months Bleomycin lung toxicity 32% (Mortality: 25%) Evens, et al. Blood 2013, 122(21). 120 150 180 Recommendations for Managing Older Adults with HL • Outcomes inferior to younger patients • Increased toxicity and treatment-related mortality • Take great caution regarding bleomycin lung toxicity and neutropenic infections • Low threshold to omit bleomycin (AVD) • Short course therapy if possible • Consider myeloid growth factor support • Non-ABVD/AVD options include ChlVPP, CHOP • Clinical trials preferred • Ongoing studies examining incorporation of brentuximab vedotin into frontline therapy Targeted Therapy and New Treatment Approaches Case Study 2 32 yo woman was diagnosed with stage IV cHL a year ago. She was treated with ABVD and achieved a complete response. Today, she presents with fatigue and enlarged right cervical lymph node measuring 2 x 2 cm. Imaging studies showed PET avid lesions above and below the diaphragm, with the largest nodal mass measuring 3 x 2 cm. Bone marrow biopsy was negative. Echocardiogram showed LVEF of 60%. Case 2: You Would Recommend: 1. Brentuximab vedotin x 16 doses followed by ASCT 2. ICE x 2-3 cycles followed by ASCT 3. BEACOPP X 6 cycles 4. DHAP x 2-3 cycles followed by ASCT 5. GND x 2-3 cycles followed by ASCT Case 2 (continued) • The patient received 3 cycles of ICE and achieved a CR. This was followed by stem cell collection, BEAM, and stem cell re-infusion (ASCT). • One year later, she had enlarged nodes above and below the diaphragm, and a biopsy of a left inguinal node confirmed the presence of relapsed HL. Case 2: You Would Recommend: 1. Brentuximab vedotin x 16 doses followed by allogeneic stem cell transplant 2. Brentuximab vedotin up to 16 doses, unless disease progression or prohibitive toxicity 3. Brentuximab vedotin x 4 then PET/CT. If CR continue for at least 8 doses and a maximum of 16 doses, followed by observation 4. Clinical trial ABVD Relapse/Refractory ICE DHAP Response No Response GVD IGEV Response Cure Brentuximab Vedotin No Response ASCT No Response/Relapse Brentuximab Vedotin Results of Salvage Pre-transplant Regimens in cHL IGEV mini-BEAM ASHAP MINE PR Dexa-BEAM ICE DHAP GVD GDP ESHAP 0 20 40 60 % response rate 80 100 ICE + ASCT Moskowitz C H et al. Blood 2001;97:616-623 Event-free Survival for Transplant-naive Patients GVD plus ASCT GVD after failing a prior transplant GVD = gemcitabine, vinorelbine, and pegylated liposomal doxorubicin Bartlett N et al. Ann Oncol 2007; 18: 1071-1079. MSKCC 11-142: Relapsed/refractory HL First TX following upfront therapy Weekly BV x 2 cycles PET + - Augmented ICE x2 cycles PET + Further treatment according to treating physician Moskowitz, A et al ASH2013, Poster # 2099. - HDT/ASCT Summary • 80% CR rate achieved with PET adapted sequential therapy with BV and augmented ICE • 30% patients avoided ICE salvage therapy • Of 40 evaluable patients – 36 have completed ASCT – 3 will undergo ASCT shortly – 1 remains on treatment for persistent disease Moskowitz, A et al ASH2013, Poster # 2099. Overall Survival by Time to Relapse After Transplant TTR >12 m 6-12 m 4-6 m 0-3 m N Median OS (y) 172 4.6 165 2.4 204 1.5 215 0.7 Date of Prep 09/10/13 EUCAN/ADC/2013-10029l Horning S et al, Ann Oncol 2008:19 (suppl 4):Abstract 118; Arai S et al. Leukaemia and Lymphoma. 2013. Targeted Therapy in Hodgkin Lymphoma Lee & Younes, Hematology 2013: 394-399. 1992 (Cell): Durkop and Stein: Molecular cloning of CD30 = TNF receptor family member Younes A & Kadin M et al: J Clin Oncol, 21, 2003: 3526-3534. Summary Results of Phase I/II Clinical Trials Targeting CD30 Drug Disease Antibody type Phase Number of evaluable patients PR CR %PR + CR MDX-060 HL, ALCL Humanized I HL = 63 ALCL = 9 2 2 2 0 6% 22% SGN-30 HL, ALCL Chimeric I 24 0 0 0 SGN-30 HL, ALCL Chimeric II HL = 38 ALCL = 41 0 5 0 2 0 17% Xmab2513 HL Humanized I 13 1 0 7% 131I-Ki4 HL Murine I 22 5 1 27% Younes, A: Curr Opin Oncol. 2011, 23(6): 587-593. Brentuximab Vedotin (SGN-35) Structure Katz J, JanikJ, and Younes A . Clin Cancer Res 2011; 17: 6428-6436 Phase-I Brentuximab Vedotin in Relapsed HL Treatment Response Investigator Assessment IRF Assessment 86% of patients achieved tumor reductions 83% of patients achieved tumor reductions Younes et al. N Engl J Med 2010; 363(19): 1812-1821. Phase I Brentuximab Vedotin in Relapsed HL • 21-year-old female • HL diagnosed 2003 – – – – ABVD + XRT to mediastinum ICE BEAMASCT HDAC-inhibitor • SGN-35 2.7 mg/kg x 8 cycles – Best clinical response: CR – CT 93% reduction, PET– PET negative Younes A, et al. N Engl J Med 2010; 363(19): 1812-1821. Brentuximab Vedotin: Pivotal Phase II trial Maximum Tumor Reduction per IRF Tumour size (% change from baseline) 100 Complete remission by PET 50 0 94% (96 of 102) of patients achieved tumor reduction –50 –100 Individual patients (n=98*) Younes et al. J Clin Oncol, 2012, 30(18): 2183-2189. Brentuximab Vedotin: Pivotal Phase II trial PFS Results By Best Response • Phase II pivotal study of brentuximab vedotin in 102 patients with relapsed/refractory HL post ASCT: PFS by best response Three-year Follow-up Data and Characterization of LongTerm Remissions from an Ongoing Phase 2 Study of Brentuximab Vedotin in Patients with Relapsed or Refractory Hodgkin Lymphoma Gopal A, et al ASH 2013 OS from ASCT by BV Karuturi, M et al ASH 2012 Brentuximab Vedotin Initial treatment vs retreatment HL sALCL N=102 Bartlet et al , ASCO 2012 N=15 N= 58 N=8 Proposed Algorithm for Treating Patients post ASCT ASCT <CR/Rel apse CR Brentuximab Vedotin Observe (Role of adjuvant BV?) CR Continue BV to 8-16 doses Relapse from CR Retreat with BV then allo-SCT <CR Consider allo SCT Continue BV until disease progression Rationale for Using HDAC Inhibitors in HL Betlevi and Younes, Hematology Am Soc Hematol Educ Program. 2013 Mocetinostat in Relapsed Hodgkin Lymphoma 31 year old female Extensive Prior Therapy Regimen Best Response ABVD PR XRT Not Eval DHAP PR Auto Transplant Not Eval IGEV Progression DHAP Progression Fludarabine/ Melphalan Progression Allo Transplant Progression Donor lymphocyte Progression MOPP Not Eval ESHAP Progression IEV Progression Younes et al Lancet Oncology 2012 Baseline PET CT (– 52% = PR) 2 months Mocetinostat in Relapsed HL: Clinical Responses 85 mg 110 mg 58% of subjects experienced tumor reduction Younes A, et al, Lancet Oncology, 2012, 12(13), 1222-1228. Panobinostat in Patients with Relapsed/Refractory HL 40 mg (fixed dose) orally, 3 times/week 0 1 2 3 4 5 6 Week Restage: If no PD Give Panobinostat until PD or tox Younes A, et al. Blood. 2009;114: Abstract 923. International Panobinostat Phase II Study in Relapsed HL 71% of patients with tumor reduction Best % Change in SPD From Baseline (index lesions only) 100 Active Discontinued 75 PD 50 25 4 patients - SD (0%) 0 -25 6 patients - off AE prior to Eval 1 1 patient - withdrew consent prior to Eval 1 -50 PR -75 5 patients with SPD < 50% had new lesions at Eval 1 -100 Younes A,Soreda A,, et al. JCO 2012 Phase I Panobinostat (LBH589) + ICE ICE Transplant LBH PET/CT Dose Level LBH589 (Panobinostat) Dose mg N -1 10 0 0 20 6 1 30 14 Results: Evaluable patients : N=21 ORR : 86%, CR: 71% and all proceeded to ASCT Oki Y, et al ASH 2013. PI3K Pathway Inhibitors GPCR RTK TNFR BCR CD19 Survival Proliferation Growth Metabolism Apoptosis Motility BAD GSK3 FOXO p53 PI 3-kinase Idelalisib IPI-145 BKM-120 XL-147 GDC-0941 GSK1059615 AKT MK-2206 XL-418 VQD002 mTORC1 Everolimus Temsirolimus Ridaforolimus AZD8055 S6K1 Younes a, ASH 2013 4EBP1 BEZ-235 BGT226 XL765 Activity of PI3K/mTOR Inhibitors in Lymphoma Pathway Drug PI3K/AKT/ mTOR Target % Response Rate in Different Histologies DLBCL FL MCL SLL/CLL T-Cell HL Everolimus mTOR 30% 50% 32% 18% 63% 42% Temsirolimus mTOR 36% 56% 38% 10% - - Idelalisib PI3K-δ - 57% 67% 72% - 12% IPI-145 PI3K-γδ 0% 67%* 67% 54% 33% 33% BAY80-6946 PI3K-αδ 13% 40% 71% 43% 50% - Updated from Younes A & Berry D. Nature Rev Clin Oncol 2012 Targeting PD1-PDL1 McDermott et al, Cancer Medicine 2013; 2(5): 662–673. PD1/PD-L1 Blocking Agents in Clinical Trials Source Target molecule PD-1 Merck MK-3475 (humanized IgG4) CureTech/Teva CT-011 (humanized IgG1) AmpliImmune/GSK BMS Genentech/ROCHE PD-L1/PDL2 AMP-224 (PD-L2/IgG1 fusion protein MDX-1106/BMS-936558 (Fully human IgG4) MDX-1105/BMS-936559 (Fully human IgG4) MPDL3280A (Engineered IgG1) Immune regulatory effects of panobinostat in patients with Hodgkin lymphoma through modulation of T-cell PD1 expression Oki Y, …Younes A: Blood Cancer Journal (2014) 4, e236. Single Agent Activity of New Agents in Post SCT Relapsed cHL Comparison to multiagent chemotherapy GVD % Response rate 100 80 60 40 20 0 Betlevi and Younes, Hematology Am Soc Hematol Educ Program. 2013 CR PR Brentuximab-Vedotin Combination Strategies HDACi PD1/PDL1 MoAb Brentuximab Vedotin Chemo therapy PI3Ki/ mTORi