Empirical and Molecular Formula
advertisement

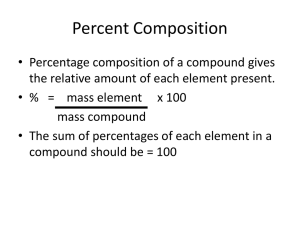
Empirical & Molecular Formulas • Law of Constant Composition a compound contains elements in a certain fixed proportions (ratios), regardless of how the compound is prepared or where it is found in nature. • If you have one molecule of methane gas, you will always have 1 carbon atoms and 4 hydrogen atoms. Empirical Formula • Empirical Formula is the formula that gives the lowest ratio of atoms in a compound. It does not necessarily tell you the exact number of each type of atom. • Example 1: The percent composition of a compound is 69.9 % iron and 30.1% oxygen. What is the empirical formula of a compound? Example 1: The percent composition of a compound is 69.9 % iron and 30.1% oxygen. What is the empirical formula of a compound? Step 1: List the given values Fe=69.9% and O = 30.1% Step 2: Calculate the mass (m) of each element in a 100g sample. mFe= 69.9 x 100g = 69.9g 100 mO= 30.1 x 100g = 30.1g 100 Step 3: Convert Mass (m) into moles (n) nFe= m/M = 69.9g/55.86g/mol = 1.25 mol Fe nO= m/M = 30.1g/16.00g/mol = 1.88 mol O Step 4: State the Amount Ratio nFe : nO 1.25mol : 1.88 mol Step 5: Calculate lowest whole number ratio 1.25mol : 1.88 mol When you don’t get a whole number, 1.25mol 1.25 mol multiply entire ratio by 2, 3, 4 etc. until you get a whole number 1 : 1.5 2 : 3 Empirical Formula is Fe2O3 Example 2: The percent composition of a compound is 21.6% sodium, 33.3% chlorine, and 45.1% oxygen. What is the empirical formula of the compound? Step 1: List the given values Cl=33.3%, Na = 21.6% and O = 45.1% Step 2: Calculate the mass (m) of each element in a 100g sample. mCl= 33.3 x 100g = 33.3g Cl 100 mNa= 21.6 x 100g = 21.6g Na 100 mO= 45.1 x 100g = 45.1g O 100 Step 3: Convert Mass (m) into moles (n) nCl= m/M = 33.3g/35.5g/mol = 0.94 mol Cl nNa= m/M = 21.6g/23.0g/mol = 0.94 mol Na nO= m/M = 45.1g/16.00g/mol = 2.82 mol O Step 4: State the Amount Ratio nFe : nNa : nO 0.94mol : 0.94mol : 2.82 mol Step 5: Calculate lowest whole number ratio 0.94mol : 0.94mol : 2.82 mol 0.94mol : 0.94mol : 0.94 mol 1 : 1: 3 Empirical Formula is NaClO3 Molecular Formula • Molecular Formula of a compound tells you exact number of atoms in one molecule of a compound. This formula may be equal to the empirical formula or may be a multiple of this formula. • To determine, you need: – The empirical formula – The molar mass of the compound Molecular Formula - shows the actual number of atoms Example: C6H12O6 Empirical Formula - shows the ratio between atoms Example: CH2O The empirical formula of a compound is CH3O and its molar mass is 93.12g/mol. What is the molecular formula? Step 1: List given values Empirical Formula=CH3O Mcompound = 93.12 g/mol Step 2: Determine the molar mass for the empirical formula, CH3O. MEmpirical = 12.01g/mol + 3(1.01g/mol) + 16.00g/mol = 31.04 g/mol Step 3. Divide the molar mass by the empirical formula molar mass. Molecular formula molar mass = 93.12 g/mol Empirical formula molar mass 31.04 g/mol =3 Step 4. Calculate Molecular Formula by multiplying this number by the empirical formula. Molecular formula = x (empirical formula) 3 x CH3O Therefore, the molecular formula is C3H9O3 Example 2: The percent composition of a compound is determined by a combustion and analyzer is a 40.03% carbon, 6.67% hydrogen, & 53.30% oxygen. The molar mass is 180.18g/mol. What is the molecular formula Step 1: List given values C= 40.03%, O=53.30%, H=6.67% Mcompound = 180.18 g/mol Step 2: Calculate the mass of each element in a 100g sample mC=40.03g mO=53.30g mH=6.67g Step 3: Convert Mass (m) into moles (n) nC= m/M = 40.03g/12.01g/mol = 3.33 mol C nH= m/M = 6.67g/1.01g/mol = 6.60 mol H nO= m/M = 53.30g/16.00g/mol = 3.33 mol O Step 4: State the Amount Ratio nC : nH : nO 3.33mol : 6.60mol : 3.33 mol Step 5: Calculate lowest whole number ratio 3.33mol : 6.60mol : 3.33 mol 3.33mol : 3.33mol : 3.33 mol 1 : 2: 1 Empirical Formula is CH2O Step 6: Determine the molar mass for the empirical formula MEmpirical = 12.01g/mol + 2(1.01g/mol) + 16.00g/mol = 30.03 g/mol Step 7. Divide the molar mass by the empirical formula molar mass. 180.18 g/mol Molar mass Empirical formula molar mass = 30.03 g/mol =6 Step 8. Calculate Molecular Formula by multiplying this number by the empirical formula. Molecular formula = x (empirical formula) 6 x (CH2O) Therefore, the molecular formula is C6H12O6 Example 3: The percent composition of a compound is determined by a combustion and analyzer is a 32.0% carbon, 6.70% hydrogen, 42.6% oxygen & 18.7% nitrogen. The molar mass is 75.08g/mol. What is the molecular formula? Calculate the mass of each element in a 100g sample mC=32.0g mO=42.6g mH=6.70g mN=18.7g Convert Mass (m) into moles (n) nC= m/M = 32.0g/12.01g/mol = 2.66 mol C nH= m/M = 6.70g/1.01g/mol = 6.65 mol H nO= m/M = 42.6g/16.00g/mol = 2.66 mol O nN= m/M = 18.7g/14.01g/mol = 1.33 mol N State the Amount Ratio nC : nH : nO : nN 2.66mol : 6.65mol : 2.6 mol: 1.33mol Step 5: Calculate lowest whole number ratio 2.66mol : 6.65mol : 2.6 mol: 1.33mol 1.33mol : 1.33mol : 1.33 mol: 1.33mol 2 : 5: 2: 1 Empirical Formula is C2H5O2N Determine the molar mass for the empirical formula MEmpirical = 75.08g Divide the molar mass by the empirical formula molar mass. Molar mass Empirical formula molar mass 75.08 g/mol 75.08 g/mol = =1 Calculate Molecular Formula by multiplying this number by the empirical formula. Molecular formula = x (empirical formula) 1 x (C2H5O2N) Therefore, the molecular formula is C2H5O2N