File - Mrs. Owens` (Stancik) Chemistry Class
advertisement

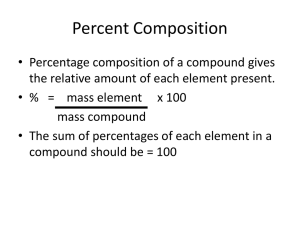
Empirical Formula 0 Consists of the symbols for the elements combined in a compound, with subscripts showing the smallest whole-number mole ratio of the different atoms in the compound EF from percentages – Step 1 0 Begin by converting % comp. to a mass composition. 0 Assume you have a 100 g sample. 0 Change % signs to grams: 0 Ex: The % composition of diborane is 78.1% B and 21.9% H. 0 Therefore, 100.0g of diborane contains 78.1g of Boron and 21.9 g of Hydrogen. EF from Percentages – Step 2 0 Convert the mass composition of each element to a molar composition. 0 Ex: 78.1 g B x 1 mol B = 7.22 mol B 10.812 g B 21.9 g H x 1 mol H 1.008 g H = 21.7 mol H We now have a mole ratio of 7.22 moles of Boron to 21.7 moles of Hydrogen However, this is NOT a ratio of SMALLEST WHOLE #s EF from Percentages – Step 3 0 To find a ratio of smallest whole numbers, divide each # of moles by the smallest number in the existing ratio. 0 Ex: 7.22 mol B 21.7 mol H : 7.22 7.22 = 1 mol B : 3.01 mol H EF from Percentages - Answer 0 Because of rounding or experimental error , a compound’s mole ratio sometimes consists of #s close to whole numbers instead of exact whole numbers. 0 Differences may be ignored and nearest whole number taken. 0 Diborane contains atoms in the ration 1B : 3H. 0 Diborane’s EF is BH3 EF – Example 2 0 Quantitative analysis shows that a compound contains 32.38% sodium, 22.65% sulfur, and 44.99% oxygen. Find the empirical formula of this compound. EF – Example 3 0 Find the empirical formula of a compound found to contain 26.56% potassium, 35.41% chromium, and the remainder oxygen. Once the EF is found, name the compound. EF from mass – Step 1 0 Sometimes mass composition is known/given instead of percent composition. 0 Convert mass composition to mole composition. 0 Ex: Calcium Bromide contains 4.00 g of Calcium and 16.00 g of Bromine. 0 4.00 g Ca x 1 mol Ca 40.078 g Ca 0 16.00 g Br x = 0.0998 mol Ca 1 mol Br 79.904 g Br = 0.2002 mol Br EF from mass – Step 2 0 Calculate the smallest whole – number mole ratio to atoms. 0 Ex: 0.0998 mol Ca 0.2002 mol Br : 0.0998 0.0998 = 1 mol Ca : 2.006 mol Br 0 Calcium Bromide’s EF is CaBr2 EF – Example 4 0 Analysis of a 10.150 g sample of a compound known to contain only phosphorus and oxygen indicates a phosphorus content of 4.433 g. What is the empirical formula of this compound? EF – Example 5 0 Analysis of a compound indicates that it contains 1.04 g K, 0.70 g Cr, and 0.86 g O. Find its empirical formula. Empirical Formula Review 0 Percent Comp. Given 0 % comp mass comp comp in moles smallest whole-number mole ratio in atoms 0 Mass Comp. given 0 Mass comp comp in moles smallest whole-number mole ratio in atoms If a .5 value occurs in the last step, multiply everything by 2 Molecular Formulas - Steps 0 1. Determine the EF 0 2. Determine the molar mass of the compound from the EF 0 3. Divide the molecular mass of the compound by the molar mass of the empirical formula 0 4. The whole number determined in step 3 is used to multiply the subscripts in the empirical formula to derive the subscripts in the molecular formula Example: 0 Analysis of a compound containing chlorine and lead reveals that the compound is 59.37% lead. The molecular mass of the compound is 349.0 g/mol. What is the molecular formula for this compound? Example 2 0 Glycerol is a thick, sweet liquid obtained as a byproduct of the manufacture of soap. Its percent composition is 39.12% carbon, 8.75% hydrogen, and 52.12 % oxygen. The molecular mass is 92.11 g/mol. What is the molecular formula for glycerol? Example 3 0 You have isolated a compound and sent it for analysis to help determine its identity. The lab sends back the results indicating that the compound is 50.00% carbon, 8.33% hydrogen, 19.44% nitrogen, and 22.22% oxygen. The molecular mass of the compound is found to be 144.0 g/mol. What is the molecular formula of the compound you isolated? Example 4 0 A component of protein called serine has an approximate molecular mass of 100.00 g/mol. If the percent composition is as follows, what is the molecular formula for serine? 0 C= 34.95% 0 H = 6.844% 0 O = 46.56% 0 N = 13.59%