Sec. V.7 REDOX Titration - Ooops!
advertisement

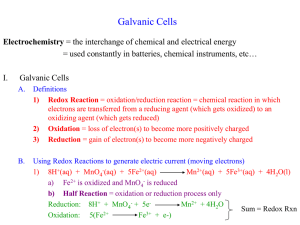
UNIT V SEC.V-7 REDOX TITRATION Redox titrations are used to determine the exact concentration of a substance that undergoes oxidation or reduction. Just like an Acid/Base titration a colour change is used to determine the endpoint of the redox reaction. Some species undergo significant colour change when being oxidized or reduced. Chemists make use of this colour change to determine the endpoint and to calculate the concentration of an unknown species. A common Oxidizing Agent is (permanganate ion) MnO4- This ion is usually made by dissolving the salt KMnO4 in water. The MnO4-(aq) is a very intense purple colour, but when it is reduced to the Mn2+ ion it becomes colourless. MnO4- + 8H+ + 5e- Mn2+ + 4 H2O Purple colour clear colour This reduction has to be done under acidic conditions (note the presence of H+) and the drastic colour change from clear to purple indicated the end point. Let’s try to see what happens when we use this oxidizing agent to oxidize Fe2+ ion to Fe3+ ion. Part 1: Preliminary Investigation of KMnO4 as an Oxidizing agent. Let’s see what colour the ions are under acidic, basic, and neutral conditions: Data Table: Type of Solution Colour Ion or Molecule Present Acidic (3 M H2SO4) clear Mn2+ Neutral (water) brown MnO2 Basic (6 M NaOH) purple MnO4- Here is the two half-cell reactions: 2 X MnO4- + 8H+ + 5e- Mn2+ + 4 H2O 5X SO32- + H2O SO42- + 2 H+ + 2 e- 2 MnO4- + 5 SO32- + 6H+ 2 Mn2+ + 5 SO42- + 3 H2O Part 2: Determining the concentration of a Solution of Fe2+ The permanganate ion will be titrated against a solution of FeSO3. The end point is reached when the colourless solution of FeSO3 remains a slight purple colour indicating that all of the Fe2+ has been reduced to Fe3+ ions. Here is the two half-cell reactions: MnO4- + 8H+ + 5e- Mn2+ + 4 H2O Fe2+ Fe3+ + 1 e- 1. Write out the balanced Redox reaction for these two half-cell reactions for acidic conditions. MnO4- + + 5 Fe2+ + 8H+ Mn2+ + 5 Fe3+ + 4 H2O 2. A 0.200 M KMnO4 solution will then be titrated against an unknown concentration of acidified FeSO3 solution. 10.0mL of FeSO3 solution will be placed into a 250 mL Erlenmeyer flask and 4.0 mL of 3 M H2SO4 is then added to this. 3. The endpoint will occur when a permanent slight purple colour appears in the Erlenmeyer flask. Data Table: Determining the Concentration of the Solution of Fe2+; Volume of KMnO4 to react with 10.0 mL of Fe2+ solution [KMnO4] = ______ Trial #1 Initial Volume of KMnO4 (mL) 0.00 Final Volume of KMnO4 (mL) Volume of KMnO4 required (mL) Average Volume (mL) Trial #2 Trial #3 (If necessary) Analysis: 1. Calculate the number of moles of the KMnO4 solution was used at the end point. n = C xV 2. How many moles of Fe2+ ions can the number of moles of KMnO4 solution oxidize? (Hint: Use your balanced REDOX reaction). 3. What was the concentration of the Fe2+ solution? 1) Do the Follow up Questions: #1 & #2 2) For more practice go to your workbook p. 213 – 214 and do exercises #26, 28, 30 and 32 3) Quiz next class on Balancing REDOX reactions and REDOX Titrations