Lab 19.1: Redox Titration: Iron and Manganese
advertisement
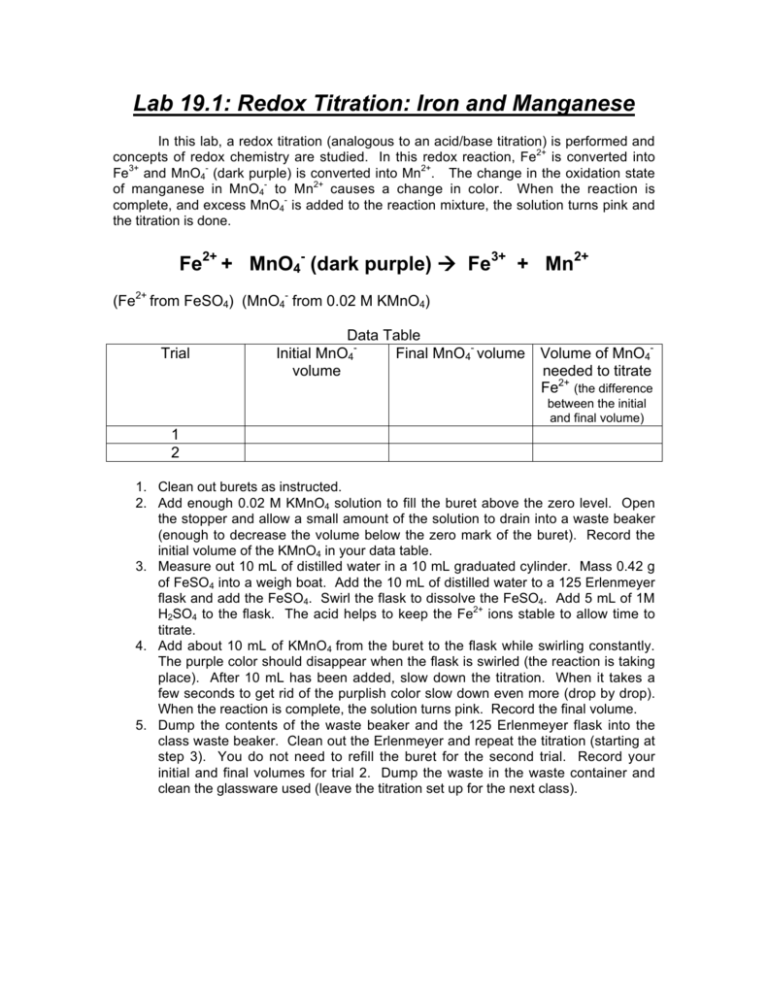
Lab 19.1: Redox Titration: Iron and Manganese In this lab, a redox titration (analogous to an acid/base titration) is performed and concepts of redox chemistry are studied. In this redox reaction, Fe2+ is converted into Fe3+ and MnO4- (dark purple) is converted into Mn2+. The change in the oxidation state of manganese in MnO4- to Mn2+ causes a change in color. When the reaction is complete, and excess MnO4- is added to the reaction mixture, the solution turns pink and the titration is done. Fe2+ + MnO4- (dark purple) Fe3+ + Mn2+ (Fe2+ from FeSO4) (MnO4- from 0.02 M KMnO4) Trial Data Table Initial MnO4Final MnO4- volume Volume of MnO4volume needed to titrate Fe2+ (the difference between the initial and final volume) 1 2 1. Clean out burets as instructed. 2. Add enough 0.02 M KMnO4 solution to fill the buret above the zero level. Open the stopper and allow a small amount of the solution to drain into a waste beaker (enough to decrease the volume below the zero mark of the buret). Record the initial volume of the KMnO4 in your data table. 3. Measure out 10 mL of distilled water in a 10 mL graduated cylinder. Mass 0.42 g of FeSO4 into a weigh boat. Add the 10 mL of distilled water to a 125 Erlenmeyer flask and add the FeSO4. Swirl the flask to dissolve the FeSO4. Add 5 mL of 1M H2SO4 to the flask. The acid helps to keep the Fe2+ ions stable to allow time to titrate. 4. Add about 10 mL of KMnO4 from the buret to the flask while swirling constantly. The purple color should disappear when the flask is swirled (the reaction is taking place). After 10 mL has been added, slow down the titration. When it takes a few seconds to get rid of the purplish color slow down even more (drop by drop). When the reaction is complete, the solution turns pink. Record the final volume. 5. Dump the contents of the waste beaker and the 125 Erlenmeyer flask into the class waste beaker. Clean out the Erlenmeyer and repeat the titration (starting at step 3). You do not need to refill the buret for the second trial. Record your initial and final volumes for trial 2. Dump the waste in the waste container and clean the glassware used (leave the titration set up for the next class). Calculations and Conclusion 1. Write the reaction (in bold at the top of the first page) into your lab notebook and assign oxidation numbers to Fe and Mn (for cations: oxidation numbers are the same as the charge on the cation). Using this information, identify the following: the element being oxidized, the element being reduced, the element that acts as the oxidizing agent, and the element that acts as the reducing agent. 2. Using the steps from class notes, write the balanced redox equation. Show all work and box the final balanced equation. 3. Calculate the volume of MnO4- needed to completely titrate Fe2+ (the difference between the initial and final volume) and put the results in the data table. Use the average volume of the two trials for step 4. 4. Using molarity = moles/L and the volume from step 3, calculate the number of moles of 0.02M MnO4- needed to completely titrate Fe2+. 5. Using the mole ratios in the balanced redox reaction, calculate the number of moles of Fe2+ in the sample. Using the equation for molarity, the number of moles of Fe2+ and the volume of the FeSO4 solution, calculate the molarity of the FeSO4 solution. NOTE: You can not take a shortcut and find the moles of Fe2+ using the molar mass of FeSO4 because of chemistry that we have not covered! 6. Write your conclusion paragraph.











