Galvanic Cells Electrochemistry = used constantly in batteries, chemical instruments, etc… I.
advertisement

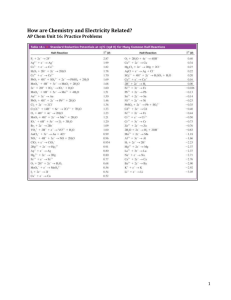
Galvanic Cells Electrochemistry = the interchange of chemical and electrical energy = used constantly in batteries, chemical instruments, etc… I. Galvanic Cells A. Definitions 1) Redox Reaction = oxidation/reduction reaction = chemical reaction in which electrons are transferred from a reducing agent (which gets oxidized) to an oxidizing agent (which gets reduced) 2) Oxidation = loss of electron(s) to become more positively charged 3) Reduction = gain of electron(s) to become more negatively charged B. Using Redox Reactions to generate electric current (moving electrons) 1) 8H+(aq) + MnO4-(aq) + 5Fe2+(aq) Mn2+(aq) + 5Fe3+(aq) + 4H2O(l) a) Fe2+ is oxidized and MnO4- is reduced b) Half Reaction = oxidation or reduction process only Reduction: 8H+ + MnO4- + 5eMn2+ + 4H2O Sum = Redox Rxn 2+ 3+ Oxidation: 5(Fe Fe + e-) C. Balancing Redox Equations: Half-Reaction Method in Acidic Solution – MnO4-(aq) + Fe2+(aq) -----> Fe3+(aq) + Mn2+(aq) (acidic solution) 1) Identify and write equations for the two half-reactions a. MnO4- -----> Mn2+ (this is the reduction half-reaction) (+7)(-2) (+2) b. Fe2+ -----> Fe3+ (this is the oxidation half-reaction) (+2) (+3) 2. Balance each half-reaction a. Add water if you need oxygen b. Add H+ if you need hydrogen (since we are in acidic solution) c. Balance the charge by adding electrons d. MnO4- -----> Mn2+ + 4H2O e. 8H+ + MnO4- -----> Mn2+ + 4H2O (+7) (+2) f. 5e- + 8H+ + MnO4- -----> Mn2+ + 4H2O g. Fe2+ -----> Fe3+ + 1eBalanced! Balanced! 3. Equalize the number of electrons in each half-reaction and add reactions a. 5(Fe2+ -----> Fe3+ + 1e-) = 5Fe2+ -----> 5Fe3+ + 5eb. 5e- + 8H+ + MnO4- -----> Mn2+ + 4H2O c. 5Fe2+ + 8H+ + MnO4- ------> 5Fe3+ + Mn2+ + 4H2O d. Species (including e-) on each side cancel out (algebra) e. Check that the charges and elements all balance: DONE! 4. Example: H+ + Cr2O72- + C2H5OH -----> Cr3+ + CO2 + H2O D. Balancing Redox Equations: Half-Reaction Method in Basic Solution 1) Follow the Acidic Solution Method until you have the final balance eqn 2) H+ can’t exist in basic solution, so add enough OH- to both sides to turn all of the H+ in H2O 3) Example: Ag + CN- + O2 -----> Ag(CN)2- (basic solution) a. Ag + CN- -----> Ag(CN)2- (oxidation half-reaction) b. Becomes: Ag + 2CN- -----> Ag(CN)2- + 1e- Balanced c. O2 -----> (reduction half-reaction) d. Becomes: 4e- + 4H+ + O2 -----> 2H2O Balanced e. f. g. h. i. Equalize the electrons in each half-reaction and add reactions 4(Ag + 2CN- -----> Ag(CN)2- + 1e-) Becomes: 4Ag + 8CN- -----> 4Ag(CN)2- + 4eAdd to: 4e- + 4H+ + O2 -----> 2H2O Gives: 4Ag + 8CN- + 4H+ + O2 -----> 4Ag(CN)2- + 2H2O DONE! j. Add OH- ions to both sides to remove H+ ions k. 4Ag + 8CN- + 4H+ + O2 + 4OH- -----> 4Ag(CN)2- + 2H2O + 4OHl. 4Ag + 8CN- + 4H2O + O2 -----> 4Ag(CN)2- + 2H2O + 4OHm. Cancel water molecules appearing on both sides of the equation 4Ag + 8CN- + 2H2O + O2 -----> 4Ag(CN)2- + 4OHn. Check that everything balances REALLY DONE! 4) 5) In solution: a) Fe and MnO4- collide and electrons are transferred b) No work can be obtained; only heat is generated In separate compartments, electrons must go through a wire = Galvanic Cell a) Generates a current = moving electrons from Fe2+ side to MnO4- side b) Current can produce work in a motor c) Salt Bridge = allows ion flow without mixing solutions (Jello-like matrix) d) E. Chemical reactions occur at the Electrodes = conducting solid dipped into the solution i) Anode = electrode where oxidation occurs (production of e-) ii) Cathode = electrode where reduction occurs (using up e-) Cell Potential 1) Think of the Galvanic Cell as an oxidizing agent “pulling” electrons off of the reducing agent. The “pull” = Cell Potential a) ecell = Cell Potential = Electromotive Force = emf b) Units for ecell = Volt = V 1 V = 1 Joule/1 Coulomb 2) Voltmeter = instrument drawing current through a known resistance to find V Potentiometer = voltmeter that doesn’t effect V by measuring it II. Standard Reduction Potentials A. Standard Hydrogen Electrode 1) When measuring a value, you must have a standard to compare it to 2) Cathode = Pt electrode in 1 M H+ and 1 atm of H2(g) Half Reaction: 2H+ + 2eH2(g) e1/2 = 0 3) We will use this cathode to find ecell of other Half Reactions B. What is the e1/2 of Zno Zn2+ + 2e1) Cathode = SHE Anode = Zn(s) in 1 M Zn2+(aq) (standard states) 2) Voltmeter reads ecell = 0.76 V 3) Define eocell = ecell at standard states so everyone can compare data 4) eocell = eo(H+ H2) + eo(Zno Zn2+) +0.76 V = 0.00 V + x 6) x = eo(Zno Zn2+) = +0.76 V 7) Combine known values in other Galvanic Cells to determine other eo1/2 values a) Zn(s) + Cu2+(aq) Zn2+(aq) + Cu(s) b) Anode: Zn Zn2+ + 2ec) Cathode: Cu2+ + 2eCu d) eocell = 1.10 V = eo(Zn) + eo(Cu2+) e) 1.10 V = +0.76 V + eo(Cu2+) f) eo(Cu2+ + 2eCu) = +0.34 V 7) Standard Reduction Potentials can be found in your text appendices a) Always given as a reduction process b) All solutes are 1M, gases = 1 atm 8) Combining Half Reactions to find Cell Potentials a) Reverse one of the half reactions to an oxidation; this reverses the sign of e1/2 b) Don’t need to multiply for coefficients = Intensive Property (color, flavor) c) Example: 2Fe3+(aq) + Cuo 2Fe2+(aq) + Cu2+(aq) i. Fe3+ + eFe2+ e1/2 = +0.77 V ii. Cu2+ + 2eCuo e1/2 = +0.34 V iii. Reverse of (ii) added to (i) = -0.34 V + +0.77 V = +0.43 V = e1/2 C. Line Notation = shorthand way to draw Galvanic Cells 1) 2Al3+(aq) + 3Mg(s) 2Al(s) + 3Mg2+(aq) 2) Line Notation: Mg(s) | Mg2+(aq) || Al3+(aq) | Al(s) a) Left side = Anode, Right side = Cathode b) | = phase change, || = salt bridge c) Far left = anodic electrode material, Far right = cathodic electrode material 3) 2MnO4-(aq) + 6H+(aq) + 5ClO3-(aq) 2Mn2+(aq) + 3H2O(l) + 5ClO4-(aq) a) Anode: ClO3- + H2O ClO4- + 2H+ + 2eb) Cathode: MnO4- +5e- + 8H+ Mn2+ + 4H2O Pt(s) Pt(s) ClO3-, ClO4-, H+ c) Mn2+, H+, MnO4- Pt(s) | ClO3-(aq), ClO4-(aq), H+(aq) || H+(aq), MnO4-(aq), Mn2+(aq) | Pt(s) D. Direction of electron flow in a cell 1) Cell always runs in a direction to produce a positive ecell 2) Fe2+ + 2eFeo e1/2 = -0.44 V MnO4- + 5e- + 8H+ Mn2+ + 4H2O e1/2 = +1.51 V 3) We put the cell together to get a positive potential a) 5(Feo Fe2+ + 2e-) b) 2(MnO4- + 5e- + 8H+ Mn2+ + 4H2O) 16H+(aq) + 2MnO4-(aq) + 5Feo(s) 4) e1/2 = +0.44 V e1/2 = +1.51 V 2Mn2+(aq) + 5Fe2+(aq) + 8H2O(l) ecell = 1.95V Line Notation: Fe(s) | Fe2+(aq) || MnO4-(aq), Mn2+(aq), H+(aq) | Pt(s) E. The complete description of a Galvanic Cell 1) Items to include in the description a) Cell potential (always +) and the balanced overall reaction b) Direction of electron flow c) Designate the Anode and the Cathode d) Identity of the electrode materials and the ions present with concentration 2) Example: Completely describe the Galvanic Cell based on these reactions Ag+ + eAg eo = +0.80 V Fe3+ + eFe2+ eo = +0.77 V Ag+(aq) + Fe2+(aq) Ago(s) + Fe3+(aq) eocell = +0.03 V Pt(s) | Fe2+(aq), Fe3+(aq) || Ag+(aq) | Ag(s)