Using Stoichiometry to Determine Chemical
advertisement

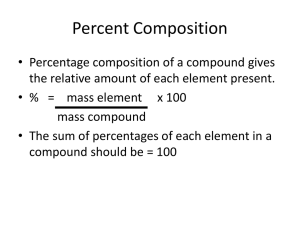
Q3U2 :Theoretical Yield, Mass Percents, and Empirical Formulas Theoretical Yield: The amount of product formed through a chemical reaction, as predicted by Stoichiometry Actual Yield: The amount of product formed when a reaction is performed in a lab, usually less than theoretical yield. Why are they different? The collection techniques, apparatus used, time, and skill of the chemist can effect the actual yield. When actual and theoretical yields are different, you express the efficiency of the reaction using percent yield. Percent yield is the ratio of actual yield to theoretical yield, expressed as a percent. Percent yield = (actual yield) X 100 (theoretical yield) Chemical formulas tell you the elements involved in a reaction, the number of atoms of each and the number of moles of reactants and products What is the major element in a reaction, by mass? To answer this, determine the mass percents of each element Steps 1. Determine the molar mass of the element in the compound 2. Multiply the mass of 1 mole times the number of moles in the compound 3. Now use that mass to find the mass percent in the sample In 154 grams of C10H18O (Geraniol), How many grams does the Carbon atom contribute? 1 molecule of C10H18O contains 10 atoms of Carbon Therefore 1 mole of C10H18O contains 10 moles of carbon atoms Multiply the mass of 1 mole of carbon by 10 to get the mass of carbon in one mole C10H18O (10 mol) (12.0gC) = 120g C (1 mol) Use mass to determine mass percent mass % of C = ( 120 gC ) X 100 = 77.9% (154 g C10H18O) Given 154 g of C10H18O What is mass% of H? 18 mol H X 1.01 g/mole = 18 g H Mass% H = mass H X 100 mass C10H18O = 18.0 g H X 100 = 11.7% H 154 g C10H18O What is the Mass% of O? What is the Mass% of O? (1 mol O) (16 g O) = 16 g O ( 1 mol) 16 g O X 100 = 10.4% O 154g C10H18O The previous steps will allow you to determine the composition of any compound, as long as you know the formula. Try the Following: 1. Hydrogen fuels are rated with respect to their hydrogen content. Determine the percent hydrogen for the following fuels. a) Ethane, C2H6 b) Methane, CH4 c) Whale Oil, C32H64O2 a) b) c) Ethane, C2H6 2 mol C, 6 mole H X 12.0g X 1.0 g 24.0g 6.0 g (6.0 g/30.0 g ) X 100 = 20% = 30.0g Methane, CH4 1 mol C , 4 mol H X 12.0g X 1.0 g 12.0 g 4.0 g (4.0 g/16.0 g ) X 100 = 25% Whale Oil, C32H64O2 32 mol C, 64 mol H, 2 mol O X 12.0g X 1.0 g X 16.0 g 384. 0g (64.0 g/480.0 g ) X 100 = 13% 64.0 g 32.0 g Calculate the Percent Composition of oxygen in the following compounds a. SO3 b. CH3COOH c. Ca(NO3)2 d. Ammonium Sulfate, (NH3) SO2 a. b. c. d. 60.00% 53.29% 58.50% 48.43% Hydrogen peroxide (H2O2), what is the mass % of Oxygen? Sodium nitrate (NaNO3), what is the mass % of Oxygen? a) H2O2 2 mol O, 2 mole H X 16.0g X 1.0 g 32.0g 2.0 g (32.0 g/34.0 g ) X 100 = 94.1% b) NaNO3 = 34.0g 1 mol Na, 1 mol N, 3 mole O X 23.0g X 14.0 g X 16.0 g 23.0 g 14.0g 48.0 g (48.0 g/85.0 g ) X 100 = 56.4% Fish in some lakes have been found to contain a mercury compound, possibly a contaminant from the making of paper. Analysis of this compound gives the following mass percentages: Carbon, 5.57% ; hydrogen, 1.40% ; and mercury, 93.03%. Using this information, determine the Empirical Formula of the compound. Empirical Formula: the formula of a compound that has the smallest whole-number ratio of atoms in the compound Steps 1. Fin d the relative numbers of atoms in the 2. 3. formula unit of the compound Use the molar mass to find the number of moles of each element Divide the mole numbers by the smallest one, to find the whole number ratios Empirical Formula: the formula of a compound that has the smallest whole-number ratio of atoms in the compound Analysis of this compound gives the following mass percentages: Carbon, 5.57% ; hydrogen, 1.40% ; and mercury, 93.03%. Using this information, determine the Empirical Formula of the compound. Carbon: 5.57g 12.01 g/mol = 0.464 mol ratio = 1 Hydrogen: 1.40 g 1.008 g/mol = 1.39 mol ratio: 1.39/ 0.464 = 3 Mercury: 93.03g 200.59 g/mol = 0.464 mol ratio = 1 Empirical formula is CH3Mg, methyl mercury An unknown compound is 18.8% Na, 29.0% Cl and 52.2% O, what is the empirical formula of the unknown? Use molar mass to find the number of moles of each element. (18.8 g Na) (1mol Na) = 0.817 mol Na (23.0 g Na) (29.0 g Cl) (1mol Cl) = 0.817 mol Cl (35.5 g Cl) (52.2 g O) (1mol O) = 3.26 mol O (16.0 g O) Divide the mole numbers by the smallest one. 0.817/ 0.817 = 1 mol Na ratio = 1 0.817/ 0.817 = 1 mol Cl ratio = 1 3.26/ 0.817 = 3.99 mol O ratio = 4 The Empirical formula is NaClO4 Hydrogen = 5.17%, nitrogen is 35.9% and sodium is 58.9%, what is the empirical formula? 5.17 g H X 1 mol H/ 1.01 g H = 5.12 mol H 35.9 g N X 1 mol N/ 14.0 g N = 2.56 mol N 58.9 g Na X 1 mol Na/ 23.0 g Na = 2.56 mol Na Ratio of H:N:Na is 2:1:1 Empirical formula is NaNH2 a. 15.8% carbon and 84.2% sulfur b. 43.6% phosphorus and 56.4% oxygen c. 28.7% K, 1.5% H, 22.8% P and 47.0% O a. b. c. CS2 P2O5 KH2PO4 Calculate the empirical formula for the following compounds from percent composition a. 0.0130 mol C, 0.0390 mol H, 0.0065 mol O b. 11.66 g iron, 5.01 g oxygen c. 40.0 percent C, 6.7 percent H, and 53.3 percent O by mass a. b. c. C2H6O Fe2O3 CH2O For most ionic compounds the two are the same Covalent compounds can be a very different case. The ratio between moles is the same, but the atoms can share electrons differently, so the formula may be different. ▪ Ex. C6H12O6 and C3H6O3 and C2H4O2 glucose lactic acid acetic acid Different compounds with different properties, same molar ratios, different chemical formulas! You need the molar mass of the compound, change to molecular mass (amu) Divide the molecular mass of the compound by the molecular mass of the empirical formula unit This will tell you how many of the empirical formula units are in the chemical formula 40.0% C, 6.7% H, 53.3% O Find the empirical formula Is this the same as the chemical formula, if the molar mass is 90.0 g/mol 3.33 mol C = 1 mol C 6.7o mol H = 2.01 mol H 3.33 mol O = 1.o mol O Empirical formula is CH2O 90.0 g/mol = 90.0 amu molecular mass CH2O molecular mass= 12+2 + 16= 30 amu 90.0/30.0 =3 3 empirical formula units C3H6O3, lactic acid a. empirical formula CH , molar mass = 78 g/mol b. empirical formula NO2 , molar mass = 92.02 g/mol c caffeine, 49.5% C , 5.15% H , 28.9% N , 16.5% O by mass, molar mass = 195 g. a. C6H6 b. N2O4 c. Use mlar mass to find ratios, and empirical formula first, divide molecular and empirical formula masses to determine number of empirical formula units in sample grams C8H10N4O2