File
advertisement

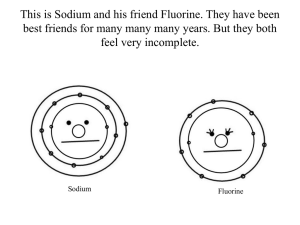
Periodic Table of the Elements: Practice Problems Group and Period PTE Group • • • • The columns on the PTE are called groups. Each group follows a pattern. All the elements in a group have the same number of valence electrons. All the elements in a group need to gain/lose/share the same number of valence electrons to achieve ideal electron configuration. • Group number (1-8) indicates the number of valence electrons. • Group 1=1 valence electron • Group 4=4 valence electrons • To achieve ideal electron configuration, an element will gain/lose electrons to achieve the number of electrons of the “nearest” Nobel Gas. Valence Electron Problem 1: • How many valence electrons does Silicon (Si) have? • It is in group 4/14, so it has 4 valence electrons. Valence Electron Problem 2: • How many valence electrons does Barium (Ba) have? • It is in group 2, so it has 2 valence electrons. Valence Electron Problem 3: • How many valence electrons does Iodine (I)have? • It is in group (7/17), so it has 7 valence electrons. Ideal Electron Configuration Problem 1: • How must the number of electrons change for Nitrogen (N) to be stable? • • • • Nitrogen is in Period 5/15. It is three “spaces” away from Neon, the nearest Noble Gas. It is five spaces away from Helium, the next closest Noble Gas. It can either gain 3 electrons or lose 5 electrons to get an ideal electron configuration. • It is more likely it will gain 3 electrons. Ideal Electron Configuration Problem 3: • How must the number of electrons change for Carbon (C) to be stable? • • • • Carbon is in Period 4/14. It is four “spaces” away from Neon, the nearest Noble Gas. It is four spaces away from Helium, the next closest Noble Gas. It can either lose 4 electrons or gain 4 electrons to get an ideal electron configuration. • It is equally likely that it will gain or lose 4 electrons. Ideal Electron Configuration Problem 3: • How must the number of electrons change for Sulfur (S) to be stable? • • • • Sulfur is in Period 6/16. It is two “spaces” away from Argon, the nearest Noble Gas. It is six spaces away from Neon, the next closest Noble Gas. It can either lose 6 electrons or gain 2 electrons to get an ideal electron configuration. • It is more likely it will gain 2 electrons. Electron Configuration and Electron Shells • As electrons get added, they start filling in available spaces in each Electron Shell. • There are 7 known electron shells, each containing several orbitals that can hold electrons. • When a shell is the valence (outer) shell, it will never hold more than 8 electrons. • The number of electrons in each shell are sometimes indicated on the PTE. • The period (row) that an element is found in indicates which shell is the valence shell. Electron Shell Problem 1 • How many electron shells does Tin (Sn) have? • It is in period 5, so it has 5 shells, the first 4 of which are full. Electron Shell Problem 2 • How many electron shells does Silicon (Si) have? • It is in period 3, so it has 3 shells, the first 2 of which are full. Electron Shell Problem 3 • How many electron shells does Copper (Cu) have? • It is in period 4, so it has 4 shells, the first 3 of which are full. Valence Shells and Electrons Problem 1: • An element has 3 valence electrons and 3 electron shells. What element is it? • If it has 3 valence electrons, it is probably from group 3/13. • If it has 3 electron shells, it must be period 3. • The matching element is Aluminum. Valence Shells and Electrons Problem 2: • An element has 7 valence electrons and 2 electron shells. What element is it? • If it has 7 valence electrons, it is from group 7/17. • If it has 2 electron shells, it must be period 2. • The matching element is Fluorine (F). Valence Shells and Electrons Problem 3: • An element has 5 valence electrons and 5 electron shells. What element is it? • If it has 5 valence electrons, it is from group 5/15. • If it has 5 electron shells, it must be period 5. • The matching element is Antimony (Sb). Simple Problem 1: • An element is in group 6/16 and period 4. What element is it? • The matching element is Selenium (Se). Simple Problem 2: • An element is in group 3/13 and period 7. What element is it? • Ununtrium Simple Problem 3: • How many electron shells and valence electrons does the element Strontium (Sr) have? • It is in period 5, so it has 5 electron shells. • It is in group 2, so it has 2 valence electrons in its valence shell. Tough Problem 1: • An element has 12 neutrons in a typical isotope, and has an ideal electron configuration identical to neon. What element is it? • If its ideal electron configuration is the same as neon’s, it must be within 4 spaces of neon. • Fluorine is not massive enough: it only has 10 neutrons. • Looking at more massive elements, sodium has a mass of 23 and an atomic number of 11. 23-11=12 neutrons. Sodium matches. • Magnesium has a mass of 24 and 12 protons. 24-12=12 neutrons. Magnesium matches. Tough Problem 2: • An element needs to gain or lose 2 electrons to gain ideal electron configuration. It has 5 filled electron shells, and one partially filled shell. What elements could it be? • It must be in period 6. • Barium could lose 2 electrons to become like Xenon, or Polonium could gain 2 electrons to become like Radon. Tough Problem 3 • An element has 81 neutrons, and an ideal electron configuration similar to Xenon. What element is it? • It must be in period 5 or 6. • Barium has an atomic mass of 137, and 56 protons. 137-56=81 neutrons. • Barium must be the answer.