empirical formula - richardkesslerhfa
advertisement

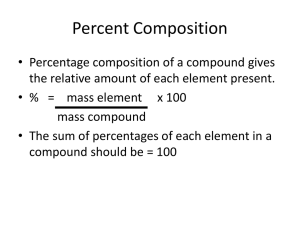
Chemical Quantities Unit 7 Chapter 10, Section 10.3 Percent Composition and Chemical Formulas Objectives • When you complete this presentation, you will be able to … – describe how to calculate the percent by mass of an element in a compound. – interpret an empirical formula. – distinguish between empirical and molecular formulas. Introduction • Chemical formulas tell us about the number of atoms in a compound. • In general, there are two kinds of chemical formulas. – In molecular formulas, the total number of atoms in the compound is used. – In empirical formulas, the lowest whole number ratio of atoms in the compound is used. Molecular Formulas • In molecular formulas, the total number of atoms in the compound is used. – For example, benzene has 6 carbon atoms and 6 hydrogen atoms in each molecule. • Therefore, its molecular formula is C6H6. Molecular Formulas • In molecular formulas, the total number of atoms in the compound is used. – For example, acetic acid has 2 carbon atoms, 4 hydrogen atoms, and 2 oxygen atoms in each molecule. • Therefore, its molecular formula is C2H4O2. Molecular Formulas • In molecular formulas, the total number of atoms in the compound is used. – For example, propane gas has 3 carbon atoms and 8 hydrogen atoms in each molecule. • Therefore, its molecular formula is C3H8. Empirical Formulas • In empirical formulas, the lowest whole number ratio of atoms in the compound are used. – For example, benzene has 6 carbon atoms and 6 hydrogen atoms in each molecule. • Therefore, its molecular formula is C6H6 and its empirical formula is CH (we divide all subscripts by 6). Empirical Formulas • In empirical formulas, the lowest whole number ratio of atoms in the compound are used. – For example, acetic acid has 2 carbon atoms, 6 hydrogen atoms, and 2 oxygen atoms in each molecule. • Therefore, its molecular formula is C2H4O2. and its empirical formula is CH2O (we divide all subscripts by 2). Empirical Formulas • In empirical formulas, the lowest whole number ratio of atoms in the compound are used. – For example, propane gas has 3 carbon atoms and 8 hydrogen atoms in each molecule. • Therefore, its molecular formula is C3H8 and its empirical formula is also C3H8 (there is no common divisor for all subscripts). Percent Composition • If we know the mass of a compound and the mass of one or more of the elements in the compound, then we can find the percent composition of those atoms in the compound. % mass of an element = mass of atoms × 100% mass of compound Percent Composition • For example, – 1.716 g of C, 0.577 g of H, and 2.286 g of O combine together to form 4.579 g of a compound. % mass C = mass of C 1.716 g × 100% = × 100% = 37.48% mass of compound 4.579 g % mass H = mass of H 0.577 g × 100% = × 100% = 12.60% mass of compound 4.579 g % mass O = mass of O 2.286g × 100% = × 100% = 49.92% mass of compound 4.579 g Sample Problem 10.9 • When a 13.60 sample of a compound containing only magnesium and oxygen is decomposed, 5.40 g of oxygen is obtained. What is the percent composition of this compound? Known: mass of compound = 13.60 g mass of O = 5.40 g mass of Mg = 13.60 g – 5.40 g = 8.20 g Unknown: %Mg = ?% %O = ?% % Mg = %O= mass of Mg 8.20 g × 100% = × 100% = 60.3% mass of compound 13.60 g mass of O 5.40 g × 100% = × 100% = 39.7% mass of compound 13.60 g Percent Composition • If we know the chemical formula of a compound, then we can find the percent composition of each of the atoms in the compound. • We use the molar mass of the compound and the average atomic masses of the atoms in the compound. percent composition = atomic mass of atoms × 100% molar mass of compound Percent Composition • For example: – the percent composition of benzene, C6H6, is: %C = 100% atomic mass of C atoms × molar mass of C6H6 %H = atomic mass of H atoms 6(1.01 g/mol) × 100% = × 100% = 7.76% molar mass of C6H6 78.12 g/ mol = 6(12.01 78.12 g/ mol g/mol) × 100% = 92.24% Percent Composition • For example: – the percent composition of acetic acid, C2H3O2, is: %C = 100% atomic mass of C atoms × molar mass of C2H4O2 %H = atomic mass of H atoms 4(1.01 g/mol) × 100% = × 100% = 6.73% molar mass of C2H4O2 60.06 g/ mol %O = atomic mass of O atoms × 100% = molar mass of C2H4O2 = 2(12.01 60.06 g/ mol g/mol) 2(16.00 60.06 g/ mol g/mol) × 100% = 39.99% × 100% = 53.28% Sample Problem 10.10 • Propane (C3H8), the fuel commonly used in gas grills, is one of the compounds obtained from petroleum. Calculate the percent composition of propane Known: molar mass of C3H8 = 44.0 g/mol mass of C = 3 × 12.0 g/mol = 36.0 g/mol Unknown: % Mg = %O= mass of H = 8 × 1.0 g/mol = 8.0 g/mol %C = ?% %H = ?% mass of C 36.0 g/mol × 100% = × 100% = 81.8% molar mass of C3H8 44.0 g/mol mass of H 8.0 g/mol × 100% = × 100% = 18% molar mass of C3H8 44.0 g/mol Percent Composition Practice Problems: find the percent composition of … 1. C6H14 %C = [(6×12.01)/(86.20)]×100% = 83.60% %H = [(14×1.01)/(86.20)]×100% = 16.40% 2. NaCl %Na = [(22.99)/(58.44)]×100% = 39.34% %Cl = [(35.45)/(58.44)]×100% = 60.66% 3. KNO3 4. CuSO4 5. FeCO3 %K = [(39.10)/(101.11)]×100% = 38.67% %N = [(14.01)/(101.11)]×100% = 13.86% %O = [(3×16.00)/(101.11)]×100% = 47.47% %Cu = [(63.55)/(159.61)]×100% = 39.82% %S = [(32.06)/(159.61)]×100% = 20.09% %O = [(4×16.00)/(159.61)]×100% = 40.10% %Fe = [(55.85)/(115.86)]×100% = 48.20% %C = [(12.01)/(115.86)]×100% = 10.37% %O = [(3×16.00)/(115.86)]×100% = 41.43% Empirical Formulas • In empirical formulas, the lowest whole number ratios of atoms in a compound is used. – Benzene, C6H6, has an empirical formula of CH. • A ratio of 6/6 = 1/1. – Acetic acid, C2H4O2, has an empirical formula of CH2O. • A ratio of 2/4/2 = 1/2/1 – Propane, C3H8, has an empirical formula of C3H8. • A ratio of 3/8 =3/8. Empirical Formulas • If we know the percent composition of a compound, then we can find the empirical formula of the compound. – First, we assume that we have 100 g of the compound and find the mass of each atom in the compound. – Second, we find the number of mols of each atom. – Third, we find the lowest whole number ratio of mols. Empirical Formulas • For example, we have a compound with 52.2% C, 13.1% H, and 34.7% O. – First, we assume that we have 100 g of the compound and find the mass of each atom in the compound. • 52.2 g of C • 13.1 g of H • 34.7 g of O Empirical Formulas • For example, we have a compound with 52.2% C, 13.1% H, and 34.7% O. – Next, we we find the number of mols of each atom. • nC = mC/MC = 52.2 g/12.0 g/mol = 4.35 mol C • nH = mH/MH = 13.1 g/1.01 g/mol = 13.0 mol H • nO = mO/MO = 34.7 g/16.0 g/mol = 2.17 mol O Empirical Formulas • For example, we have a compound with 52.2% C, 13.1% H, and 34.7% O. – Finally, we find the lowest whole number ratio of mols. • nC/nO = 4.35 mol/2.17 mol = 2/1 • nH/nO = 13.0 mol/2.17 mol = 6/1 – This means that there is a ratio of 2:6:1 for C:H:O • The empirical formula is C2H6O Empirical Formulas • For example, we have a compound with 44.9% K, 18.4% S, and 36.7% O. – First, we assume that we have 100 g of the compound and find the mass of each atom in the compound. • 44.9 g of K • 18.4 g of S • 36.7 g of O Empirical Formulas • For example, we have a compound with 44.9% K, 18.4% S, and 36.7% O. – Next, we we find the number of mols of each atom. • nK = mK/MK = 44.9 g/39.1 g/mol = 1.15 mol K • nS = mS/MS = 18.4 g/32.1 g/mol = 0.573 mol S • nO = mO/MO = 36.7 g/16.0 g/mol = 2.29 mol O Empirical Formulas • For example, we have a compound with 44.9% K, 18.4% S, and 36.7% O. – Finally, we find the lowest whole number ratio of mols. • nK/nS = 1.15 mol/0.573 mol = 2/1 • nO/nS = 2.29 mol/0.573 mol = 4/1 – This means that there is a ratio of 2:1:4 for K:S:O • The empirical formula is K2SO4 Sample Problem 10.11 • A compound is analyzed and found to contain25.9% nitrogen and 74.1% oxygen. What is the empirical formula of the compound? – First, we assume that we have 100 g of the compound and find the mass of each atom in the compound. • mN = 25.9 g • mO = 74.1 g Sample Problem 10.11 • A compound is analyzed and found to contain25.9% nitrogen and 74.1% oxygen. What is the empirical formula of the compound? – Next, we find the number of mols of each atom. • nN = mN/MN = 25.9 g/14.0 g/mol = 1.85 mol • nO = mO/MO = 74.1 g/16.0 g/mol = 4.63 mol – Finally, we find the lowest whole number ratio of mols. • nO/nN = 4.63 mol/1.85 mol = 2.5/1 = 5/2 – This means that there is a ratio of 5:2 for O:N • The empirical formula is N2O5 Empirical Formulas Practice problems: find the empirical formulas of compounds that have the following percent compositions. 1. 11.21% H and 88.79% O H2O 2. 92.24% C and 7.76% H CH 3. 39.99% C, 6.73% H, and 53.28% O CH2O 4. 24.27% C, 4.08%H, and 71.65% Cl CH2Cl 5. 39.96% N, 14.40% H, and 45.64% O NH5O Molecular Formulas • In molecular formulas, the total number of atoms in the compound is used. – Benzene has a molecular formula of C6H6. – Acetic acid has a molecular formula of C2H4O2. – Propane has a molecular formula of C3H8. Molecular Formulas • If we know the empirical formula of a compound, then we can find the molecular formula of the compound, if we know the molar mass of the compound. – First, we determine the empirical mass of the compound. – Second, we divide the molar mass by the empirical mass to get our multiplier. – Third, we multiply the subscripts of the empirical formula by the multiplier to get the molecular formula. Molecular Formulas • For example, we have a compound with a molecular formula of CH2O and a molar mass of 180.16 g/mol. What is the molecular formula of the compound? – First, we determine the empirical mass of the compound. EM = (1×12.01) + (2×1.01) + (1×16.00) = 30.03 Molecular Formulas • For example, we have a compound with a molecular formula of CH2O and a molar mass of 180.16 g/mol. What is the molecular formula of the compound? – Next, we divide the molar mass by the empirical mass to find the multiplier. M/EM = (180.16)/(30.03) = 5.999333999 ≈ 6 Molecular Formulas • For example, we have a compound with a molecular formula of CH2O and a molar mass of 180.16 g/mol. What is the molecular formula of the compound? – Finally, we multiply the subscripts of the empirical formula by the multiplier to get the molecular formula. CH2O ⇒ C6H12O6 Sample Problem 10.12 • Calculate the molecular formula of a compound whose molar mass is 60.0 g/mol and empirical formula is CH4N. – First, we determine the empirical mass. • EM = (1×12.0) + (4×1.0) + (1×14.0) = 30.0 – Next, divide molar mass by empirical mass. • M/EM = (60.0)/(30.0) = 2 – Finally, multiply to get the molecular formula. • CH4N ⇒ C2H8N2 Molecular Formulas Practice problems: find the molecular formulas of compounds that have the following empirical formulas and molar masses. 1. CH2O; M = 60.0 g/mol C2H4O2 2. CH2; M = 42.1 g/mol C3H6 3. NaCO2; M = 134.0 g/mol Na2C2O4 4. CH2Cl; M = 98.96 g/mol C2H4Cl2 5. NH5O; M = 35.06 g/mol NH5O