How to Find an Empirical Formula
advertisement
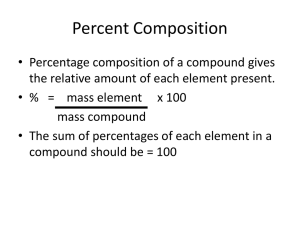
Mark S. Cracolice Edward I. Peters www.cengage.com/chemistry/cracolice Chapter 7 Chemical Formula Relationships Mark S. Cracolice • The University of Montana Number of Atoms in a Formula In writing the formula of a substance, subscript numbers are used to indicate the number of atoms or groups of atoms of each element in the formula unit. Number of Atoms in a Formula How many atoms of each element are in a formula unit of ammonium carbonate? The formula of the ammonium ion is NH4+ The formula of carbonate is CO32– The formula of the ammonium carbonate is (NH4)2CO3. Each element in the parentheses is multiplied by two. Total number of atoms of each element: 2 nitrogen atoms, 8 hydrogen atoms, 1 carbon atom, 3 oxygen atoms Atomic Mass Atomic Mass The average mass of atoms of an element By definition the mass of a carbon-12 atom is 12 u. Molecular & Formula Mass Molecular Mass The sum of the atomic masses of each atom in the molecule. Formula mass The sum of atomic masses in the formula unit Molecular & Formula Mass What is the formula mass of calcium phosphate? Calcium ion: Ca2+ Phosphate ion: PO43– Calcium phosphate: Ca3(PO4)2 Ca 3 × 40.08 u = 120.24 u P 2 × 30.97 u = 61.94 u O 8 × 16.00 u = 128.00 u Ca3(PO4)2 310.18 u Defenition of Mole The mole is the amount of substance that contains as many elementary entities as there are atoms in exactly 12 grams of carbon-12. In 12 g of carbon-12 there are 6.022 x 10 23 atoms 1 mole of any substance = 6.022 x 10 23 units of that substance. When the mole is used the elementary entities must be specified: atoms, molecules, ions… Avogadro’s Number NA The number of elementary units in one mole 6.02214179 1023 units/mol The Avogadro constant is a conversion factor between units and mole. Conversion of Mole to Molecules How many carbon dioxide molecules are in 2.0 moles of carbon dioxide? 6.02 ´ 1023 molecules CO2 2.0 mol CO2 × = mol CO2 1.2 × 1024 molecules CO2 Molar Mass Molar mass of a substance is the mass in grams of one mole of the substance. Units: g/mol Molar mass of an element is the mass of the element per mole of its atoms. Atomic mass unit and gram The mass of one atom of carbon-12 is exactly 12 atomic mass units. The mass of one mole of carbon-12 atoms (6.022 x 1023 atoms of carbon-12) is exactly 12 grams (6.022 x 1023 atoms) x (12 u/atom) = 12 grams 6.022 x 1023 u = 1 gram Molar Mass The mass of one atom of carbon-12 is exactly 12 atomic mass units. The mass of one mole of carbon-12 atoms is exactly 12 grams. This leads to the conclusion: The molar mass of any substance in grams per mole is numerically equal to the atomic, molecular or formula mass of that substance in atomic mass units. Molar Mass Calculate the mass of one NH3 molecule and the mass of one mole of NH3 molecules. 14.01 u + 3x1.008 u = 17.03 u The mass of one ammonia molecule is 17.03 u. To change from molecular mass to molar mass, change the units from u to g/mol: 17.03 g/mol. One mole of ammonia molecules has a mass of 17.03 g. Molar Mass Mass, # of Moles, # of Units Molar mass, MM, links mass in grams with the number of moles. Avogadro’s number, NA, links the number of moles with the number of particles. Mass, # of Moles, # of Units How many molecules are in 454 g of water? g moles 1 mol H O 454g x 2 18.02 g H O 2 x molecules 6.02 10 23 molecules mol H O = 1.52 x 1025 molecules H2O 2 H O 2 Mass↔Moles↔ Units How many hydrogen atoms are in 1.0 kg of ammonia? 1000 g NH3 1 mol NH3 1.0 kg NH3 × kg NH ×17.03 g NH 3 3 × 6.02 ´ 1023 molecules NH3 × 3 atoms H molecule NH3 mol NH3 = 1.1 × 1026 atoms H Percentage Composition Percentage %A= parts of A total parts × 100% The percentage composition of a compound is the percentage by mass of each element in the compound. Percentage Composition Determine the percentage composition of calcium fluoride Ca F2. Solution: In one mole of Ca F2 1x(40.08 g Ca) + 2x(19.00 g F) = 78.08 g CaF2 (Solution continued on the next slide) Percentage Composition (40.08 g Ca) 78.08 g CaF 100 = 51.33 % Ca 2 2x(19.00g F) 78.08 g CaF 100 = 48.67% F 2 Check: 51.33% + 48.67% = 100.00% Empirical Formula Empirical Formula The simplest ratio of atoms of the elements in a compound. The empirical formula of C2H4 is CH2. Likewise, the empirical formula of C3H6 is CH2. All compounds with the general formula CnH2n have the same empirical formula and therefore the same percentage composition. Empirical Formula Write the empirical formulas of benzene, C6H6, and octane, C8H18. Look for the simplest whole-number ratio of elements: For C6H6, the 6/6 ratio can be reduced to 1/1: CH. For C8H18, the 8/18 ratio can be divided by 2 on top and bottom to be reduced to 4/9: C4H9. Find Empirical Formula To find empirical formula, you need to find the ratio of atoms of the elements ratio of atoms = ratio of moles of atoms How to Find an Empirical Formula 1. Find the masses of different elements in a sample of 2. 3. 4. 5. the compound. Convert the masses into moles of atoms. Determine the ratio of moles of atoms. Express the moles ratio as the smallest possible ratio of integers. Write the empirical formula, using the number in the integer ratio as the subscript in the formula. Find Empirical Formula What is the empirical formula of a compound that has 85.6% carbon, 14.4 % hydrogen? Solution: It is usually helpful to organize the calculations in a table with the following headings: Element Grams Moles Mole Ratio Formula Ratio Empirical Formula Find Empirical Formula Element Grams C 85.6 Moles 85.6 g C 12.01 g/mol C 7.13 H 14.4 14.4 g H 1.008 g/mol H 14.3 Mole Ratio Formula Ratio 7.13 7.13 Empirical Formula 1 1 14.3 7.13 2.01 2 CH2 Find Empirical Formula What is the empirical formula of a compound that analyzes as 20.0% carbon, 2.2% hydrogen, and 77.80% chlorine? Element Grams Moles Mole Ratio Formula Ratio C 20.0 1.67 1 3 H 2.2 2.2 1.3 4 Cl 77.8 2.19 1.31 4 Empirical Formula C3H4Cl4 Molecular Formula The molecular formula of a compound can be found by determination of the number of empirical formula units in the molecule. molar mass of compound molar mass of empirical formula How to Find the Molecular Formula 1. Determine the empirical formula of the compound. 2. Calculate the molar mass of the empirical formula unit. 3. Divide the molar mass of the compound by the molar mass of the empirical formula unit to get n, the number of empirical formula units per molecule. Molecular Formula What is the molecular formula of a compound with the empirical formula C2H5and a molar mass of 58.12 g/mol? The molar mass of the empirical formula unit is 2(12.01 g/mol C) + 5(1.008 g/mol H) =29.06 g/mol The number of empirical formula units per molecule is 58.12 g/mol 29.06 g/mol =2 (C2H5)2 = C4H10 Find Molecular Formula • An unknown compound is found to be 40.0% of carbon, 6.71% of hydrogen and the remainder is oxygen. The molar mass of the compound is 180.16 g/mol. Find the empirical and molecular formulas of the compound. First Find Empirical Formula Element Grams Moles Mole Formula Empirical Ratio Ratio Formula C 40.0 3.33 1 1 H 6.71 6.66 2 2 O 53.3 3.33 1 1 CH2O Calculate Molar Mass/Empirical Formula Mass The molar mass of the empirical formula unit is (12.01 g/mol C) + 1(1.008 g/mol H) + (16.00 g/mol O) = 30.03 g/mol The number of empirical formula units per molecule is 180.16 g/mol = 6 30.03 g/mol (CH2O) 6 = C6H12O6 Molecular Formula Another way to find molecular formula is to consider one mole of the compound (180.16 g of compound). The numbers of moles are also numbers of atoms in the molecule. The masses of carbon, hydrogen and oxygen are : 40.0% x 180.16 g = 72.06 g of C 6.71% x 180.16 g = 12.09 g of H 53.3% x 180.16 g = 96.03 g of O Molecular Formula Element Grams Moles Molecular Formula C 72.06 6 H 12.09 12 O 96.03 6 C6H12O6 Homework 7, 15, 21, 23, 43, 57, 61, 63, 65, 68, 74.