Structure of Atoms
advertisement

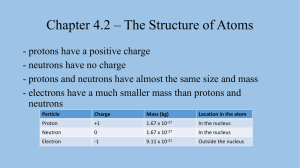
Structure of Atoms Physical Science Chapter 4 – Section 2 State Standards • CLE.3202.1.2 – Describe the structure and arrangement of atomic particles What is an Atom? • The three main components of an atom are distinquished by mass, charge, and location • The chemistry of each element depends directly on these three components • Nucleus: – Protons ( positively charged ) – Neutrons ( neutral – NO CHARGE ) • Electrons ( negatively charged ) Electric Forces in Atoms • Protons and electrons attract each other • Electric force holds components together; the same forces hold solids and liquids together • Liquid water is held together by electric forces Atomic Components • Protons ( +1 charge / 1.67 x 10-27 kg ) • Neutrons ( 0 charge / 1.67 x 10-27 kg ) • Electrons ( -1 charge / 9.11 x 10-31 kg ) • All ( most ) elements contain all three • Atoms of the same element contain the same number of protons, but neutrons may vary • Each element has a unique number of protons Distinguishing Elements and Atoms • Atomic number – number of protons in an atom • Mass number – number of protons & neutrons • Isotopes – exist for an element if different atoms ( with the same atomic number ) have varying numbers of protons ( different mass numbers ) Hydrogen & Helium • Hydrogen ( H ) contains 1 electron, 1 proton, and 0 neutrons [ Protium isotope ] • Helium ( He ) contains 2 electrons, 2 protons, and 2 neutrons • What is atomic number, mass number of H, He? Isotopes of Hydrogen • Protium – Most common form on Earth and Sun – 1 electron, 1 proton, 0 neutrons • Deuterium – small fraction of hydrogen; 1 out of every 6,000 hydrogen atoms in Earth’s crust – 1 electron, 1 proton, 1 neutron • Trillium – very unstable and so very rare – 1 electron, 1 proton, 2 neutrons Isotopes of Chlorine • The common form of chlorine ( Cl ) has 17 protons, 17 electrons, and 18 neutrons • Another form has 20 neutrons – Neutrons can be calculated by using these • How do we know which form is most common? Atomic Mass Units • Used to express the mass of such tiny particles • Unified Atomic Mass Unit is 1/12 the mass of a Carbon-12 – Carbon-12 isotope has a fairly even mass number – 6 protons and 6 neutrons – Remember electrons contribute little mass to atom – Each proton/neutron has mass of 1.0 u – Units given as a u Atomic Mass Units • The value of AVERAGE ATOMIC MASS found in the periodic table tells us which isotope of any element is most common ( HYDROGEN ) – weighted average so most common form counts most • Zinc with average atomic mass 65.4 u – 65 ( closest mass # ) – 30 ( atomic # ) = 35 neutrons – Most common Zn isotope has 35 neutrons • Cl has average of 35.453 u , with 35 mass number – Most common Cl form contains 18 neutrons The Mole • In order to count large numbers of small particles, a COUNTING UNIT was devised – THE MOLE ( mol ) • Examples ( Avogadro’s number ): – 1 mol = 602,213,670,000,000,000,000,000 particles – Equals 6.022 x 1023 when put into scientific notation – 1 mol of marbles = 6.022 x 1023 marbles – 1 mol of atoms = 6.022 x 1023 atoms ( any atom ) – 1 mol of He atoms = 6.022 x 1023 He atoms The Mole • How many stars in the universe? – 2003, roughly 7 x 1022 stars counted ( within range – Value could increase in future • 6.022 x 1023 popcorn kernels would cover the USA in a pile 500 km ( or 310 mi ) tall!! • Avogadro’s number is used only for small particles Relating Moles and Grams • Mass of 1 mol random atoms difficult to find – Each element has a unique MOLAR MASS – The mass of 1 mol of this type of atom ( element ) – Usually accounts for several isotopes • Mass of 1 mol of the same atom easy to find • For Carbon-12, molar mass is 12.00 g – Molar mass ( in g ) equals average atomic mass ( in u ) – Carbon-12 has 12.01 u as well as 12.01 g/mol • Figure 7 in text Relating Moles and Grams • Mass of 1 mol Carbon-12 atoms is 12.01 g – USE PERIODIC TABLE • 24 g Carbon-12 = 2 mol Carbon-12 • 3 mol Carbon-12 = 36 g Carbon-12 Molar Mass of Compounds, Molecules • As elements ( atoms with same atomic number ) have a MOLAR MASS, so do coumpounds • H2O : 2 Hydrogen with 1.00 g/mol & 1 Oxygen with 16 g/mol – Gives ( 2.00 + 16 ) g/mol = 18 g/mol H O • CO2 : 1 Carbon with 12.01 g/mol & 2 Oxygens with 16 g/mol – Gives ( 12.01 + 32 ) g/mol = 44 g/mol • O2 : 2 Oxygen atoms at 16 g each – Gives: 32 g/mol for O2 molecules