The Atom
advertisement

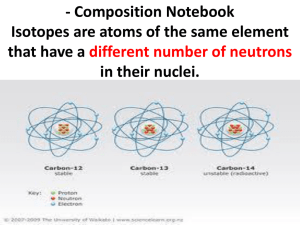
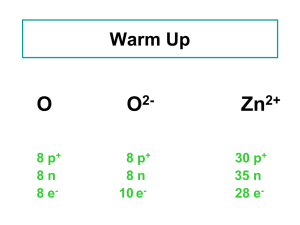
The Atom Mr. McMartin Beta Pod Science How Small is an Atom? A PENNY contains about 2 x 10Ε22 atoms or 20,000,000,000,000,000,000,000 That’s 20 thousand billion billion atoms- over 3,000,000,000,000 times more atoms than there are people on earth. An aluminum atom has a diameter of about . 00000003 cm. What is the Nucleus Made Of? Protons: positively charged particles in the nucleus. The mass of a proton is about 1.7 x 10E -24g. Because atoms masses are so small scientists made a new unit for them. Protons = 1amu Neutrons: particles of the nucleus that have no charge. Neutrons= 1amu Atomic mass unit (amu): the SI unit used to express the masses of particles in atoms. What’s Outside the Nucleus? Electrons are found outside the nucleus in the electron clouds. They are negatively charged. It takes 1,800 electrons to equal the mass of one proton. They are so small they are usually thought to have a mass of almost zero. Ion(s) The charges of protons and electrons are opposite but equal, so their charges cancel out. Because an atom has no overall charge, it is neutral. If the numbers of protons and electrons are not equal, then the atom becomes a charged particle called an ION. An atom that loses one or more electrons is a positively-charged ion. An atom that gains one or more electrons becomes a negatively-charged ion. How Do Atoms of Different Elements Differ? There are more than 110 different elements… they are all different in some way. You can tell the difference between atoms of separate elements by the number of protons in the nucleus of an atom (atomic number). Atomic number: the number of protons in the nucleus of an atom. Isotopes Isotope: atoms that have the same number of protons but have different numbers of neutrons. You can have an isotope of the same atom. Ex. Hydrogen atom vs Hydrogen Isotope Hydrogen atom= 1proton & 1electron Hydrogen isotope= 1proton, 1nutron, & 1 electron Properties of Isotopes Each element has a limited number of isotopes that are found in nature. Some isotopes have special properties because they’re unstable. These are RADIOACTIVE. Radioactive atoms spontaneously fall apart after a certain amount of time. As they do, they give off smaller particles, as well as energy. Properties of Isotopes cont. Isotopes of an element share most of the same chemical and physical properties. For example, the most common oxygen isotope has 8 neutrons in the nucleus. Other isotopes of oxygen have 9 or 10 neutrons. All three isotopes are colorless, odorless gasses at room temperature. Each isotope has the chemical property of combining with a substance as it burns. Different isotopes of an element even behave the same in chemical changes in your body. Telling Isotopes Apart You can tell each isotope apart by it’s mass number. Mass Number: the sum of the protons and neutrons in an atom. Electrons are not included in an atom’s mass number because their mass is so small. Naming Isotopes You can tell the difference between isotopes by finding how many neutrons they have. To identify a specific isotope of an element, write the name of the element followed by a hyphen and the mass number of the isotope. Ex. Hydrogen-1 or carbon-12 You can find the number of neutrons in an element by using the following equation: Mass number – Atomic number = number of neutrons Example of Finding Amount of Neutrons Find the amount of neutrons in Carbon-12. It has an atomic number of 6. Step 1: Identify mass number (12) Step 2: Identify atomic number (6) Step 3: Plug into equation 12 mass number – 6 atomic number = Step 4: solve equation to find amount of neutrons 12 mass number – 6 atomic number = 6 neutrons Calculating the mass of an element Most elements contain a mixture of two or more isotopes. Ex. All copper is composed of copper-63 atoms and copper-65 atoms. Atomic mass: the weighted average of the masses of all the naturally occurring isotopes of that element. To find the atomic mass: Multiply the mass number of each isotope by it’s percentage abundance in decimal form. Add these amounts together to find the atomic mass. Example of Finding Atomic Mass Chlorine-35 makes up 76% of all the chlorine in nature, and chlorine-37 makes up the other 24%. What is the atomic mass of chlorine? Step 1: Multiply the mass number of each isotope by it’s percentage abundance in decimal form. (35 x 0.76) = (37 x 0.24) = Step 2: Add these answers of the amounts together to find the atomic mass. DON’T FORGET TO LABEL YOUR ANSWER IN AMU’s. Forces of Atoms Forces are “pushes” and “pulls.” You have seen the make-up of individual atoms but we have not discussed the forces acting between more than one atom. There are four basic forces at work everywhere between and around an atom. Gravitational force Electromagnetic force Strong force Weak force These forces work together to give atoms their structure an properties. Gravitational Force Gravitational force acts between all objects all the time. The amount of gravitational force between objects depends on their masses and the distance between them. Because the masses of particles in atoms are so small, the gravitational force within atoms is very small. Electromagnetic Force Objects that have the same charge repel each other, while objects with opposite charge attract each other. This is due to electromagnetic force. Protons and electrons are attracted to each other because they have opposite charges. The electromagnetic force holds the electrons around the nucleus. Strong Force Protons push away from one another because of the electromagnetic force. A nucleus containing two or more protons would fly apart if it were not for the STRONG FORCE. At the close distances between protons and neutrons in the nucleus, the strong force is greater than the electromagnetic force, so the nucleus stays together. Weak Force The WEAK FORCE is an important force in radioactive atoms. In certain unstable atoms the neutron can change into a proton and an electron. The weak force plays a key role in this change.