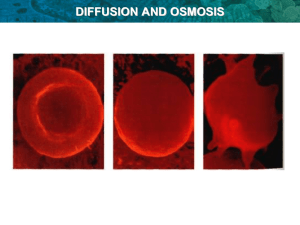
Lab 4: Diffusion and Osmosis
advertisement

Lab 4: Diffusion and Osmosis First you must review Water Potential on the next however many slides… Chapter 36 – Plant form and function AIM: Describe the process of passive transport (diffusion across the membrane). PROBLEM – What about physical pressure? physical pressure. It is easy to say that water will move from high concentration to low concentration…but look at the cell to the right…can the water keep going in? NO, as water enters and the cytoplasm begins to put pressure on the cell wall, the cell wall will begin push back (3rd law of motion) – physical pressure. The combined effects of solute concentrations and physical pressure are given in a single measurement called the WATER POTENTIAL (Ψ; psi) for a given solution (EACH SOLUTION IS ASSIGNED A WATER POTENTIAL). Chapter 36 – Plant form and function AIM: Describe the process of passive transport (diffusion across the membrane). WATER POTENTIAL How is water potential calculated? Ψ = Ψs + Ψp Ψ = water potential of the given solution 1. The potential pressure, in Mpa, that the water will exert on another solution. 2. Water always move from solutions of higher water potential (higher pressure) to solutions of lower water (lower pressure) of course. 3. If two solutions are at equilibrium then their water potential is the same… physical pressure. Chapter 36 – Plant form and function AIM: Describe the process of passive transport (diffusion across the membrane). WATER POTENTIAL physical pressure. How is water potential calculated? Ψ = Ψs + Ψp Ψ = water potential of the given solution Ψs = solute potential (osmotic potential) 1. Ψs = defined as 0 MPa for pure water; it becomes more and more negative as more and more solute is added since the potential pressure the water would exert on a neighboring solution should less if the water concentration is falling. 2. Always either zero or negative 3. Makes sense since the more solute, the more likely you are to bring water to you and therefore the lower the potential pressure exerted by the water. Chapter 36 – Plant form and function AIM: Describe the process of passive transport (diffusion across the membrane). WATER POTENTIAL physical pressure. How is water potential calculated? Ψ = Ψs + Ψp Ψ = water potential of the given solution Ψs = solute potential (osmotic potential) Ψp = [physical] pressure potential 1. Ψp = potential pressure the water will apply on a neighboring solution due to physical forces 2. Ψp can be positive or negative since the physical pressure can be greater than atmospheric pressure (positive; i.e. in a turgid plant cell) or less than atmospheric pressure (negative; i.e. xylem cells when water is flowing through by transpiration). Chapter 36 – Plant form and function AIM: Describe the process of passive transport (diffusion across the membrane). Quantitative analysis of WATER POTENTIAL Look at the four conditions on the right and explain what you are observing in terms of water potential. Water is moving from higher water potential to lower water potential whose values are dependent on solute potential and pressure potential. More solute, more negative Ψs More pressure increases Ψp Less pressure decreases Ψp Chapter 36 – Plant form and function AIM: Describe the process of passive transport (diffusion across the membrane). Quantitative analysis of WATER POTENTIAL Without looking, determine values that make sense for solute potential and pressure potential. Cytosol = 0.2M Chapter 36 – Plant form and function AIM: Describe the process of passive transport (diffusion across the membrane). Quantitative analysis of WATER POTENTIAL Cytosol = 0.2M Notice that Ψs of the cell doesn’t really change since it doesn’t take much water to enter, pressurize the cell resulting in the cell wall pushing back with the equivalent potential of 0.7 MPa. Chapter 36 – Plant form and function AIM: Describe the process of passive transport (diffusion across the membrane). Quantitative analysis of WATER POTENTIAL 1. A solution in a beaker has sucrose dissolved in water with a solute potential of -0.5MPa. A flaccid cell is placed in the above beaker with a solute potential of -0.9MPa. a) What is the pressure potential of the flaccid cell before it was placed in the beaker? 0 MPa since the cell wall applied no pressure if flaccid b) What is the water potential of the cell before it was placed in the beaker? Ψs + Ψp = Ψ -0.9 + 0 = -0.9MPa c) What is the water potential in the beaker solution containing the sucrose? Ψs + Ψp = Ψ -0.5 + 0 = -0.5MPa d) How will the water move? From high to low water potential (from beaker to cell) e) What is the pressure potential of the plant cell when it is in equilibrium with the sucrose solution outside? Also, what is its final water potential when it is in equilibrium? Equilibrium tells you that the water potential of both solutions must be the same. If the outer solution is -0.5MPa then the cytoplasm must also be -0.5MPa. Ψ = Ψs + Ψp -0.5MPa = -0.9 MPa + Ψp Ψp = 0.4, a positive number makes sense since the cytoplasm’s potential to apply pressure is increased if the cell wall is pushing on it. f) Is the cell now turgid/flaccid/plasmolysed? turgid g) Is the cell hypotonic or hypertonic with respect to the outside? It is still hypertonic since not much water would have entered before pressurizing the cell causing the membrane to push back. Lab 4: Diffusion and Osmosis How can one calculate the solute potential (ψs) for a given solution? ψs = -iCRT i = ionization constant (number of particles/ions formed when dissolved in water – ex. Glucose is 1, NaCl 2, CaCl2 3, etc…) C = Molar concentration R = pressure const. = .0831 Liter-bars/mole-K T = temperature (in Kelvin = 273 + °C) Ex. What is the solute potential for a 0.15M solution of sucrose at atmospheric pressure and a temperature of 25°C? = -(1)(.15)(.0831)(25+273) = -3.7 bars ***“bar” is a unit of pressure like “atmosphere”, “Torr” and “Pascal” (Pascal is the SI Unit). ***1bar = 100,000Pa = 100KPa = ~1atmosphere (760 Torr). Lab 4: Diffusion and Osmosis How can one calculate the solute potential (ψs) for a given solution? ψs = -iCRT i = ionization constant (number of particles/ions formed when dissolved in water – ex. Glucose is 1, NaCl 2, CaCl2 3, etc…) C = Molar concentration R = pressure const. = .0831 Liter-bars/mole-K T = temperature (in Kelvin = 273 + °C) Ex. What is the solute potential for a 0.15M solution of NaCl at atmospheric pressure and a temperature of 25°C? = -(2)(.15)(.0831)(25+273) = -7.4 bars ***“bar” is a unit of pressure like “atmosphere”, “Torr” and “Pascal” (Pascal is the SI Unit). ***1bar = 100,000Pa = 100KPa = ~1atmosphere (760 Torr). Lab 4: Diffusion and Osmosis Explain this: Lab 4: Diffusion and Osmosis Explain this: Lab 4: Diffusion and Osmosis Answer These Questions: Water and theBasis fitness the environment Chapter 23 -– The Chemical of ofLife AIM: How does one determine the pH of a solution? Recall phenolphthalein: PINK COLORLESS The structure of the phenolphthalein molecule changes in different pH values. Above pH 8, it has a structure that reflects pink. Below 8 the structure changes and does not absorb light. Structure determines function!! Lab 4: Diffusion and Osmosis Answer these questions 1. Obviously, .1M HCl is the acid as it will donate protons to solution and drop the pH 2. The base is the .1M NaOH because it will donate -OH (hydroxide ions) to solution, which will combine with H+ (protons) to form water thereby lower the [H+] and increasing the pH. 3. I think this was supposed to say what color is the phenolphthalein (the “dye”) in the acid. Adding HCl will drop the pH below 8 and therefore the solution will be clear. 4. Adding NaOH will bring the phenolphthalein solution above pH 8 and therefore it will turn pink. Lab 4: Diffusion and Osmosis The one with the highest surface area to volume ratio of course…(the small one) SA:V ratio 3:1 6:1 15:1 Agar Blocks (its just like jell-o) Design an experiment to test the hypothesis we came up with in question Lab 4: Diffusion and Osmosis Experimental Design Independent Variable:The surface area of the cubes The efficiency of the agar blocks as determined by the amount of solution that Dependent Variable: diffused into the block over a given amount of time. Procedure: 1. Make agar blocks containing phenolphthalein of assorted sizes: -2 cm3 -1 cm3 -0.5 cm3 2. The cubes start off with a pH below 8 and are therefore a white color as shown above right. Place the cubes in NaOH solution for 10 minutes. 3. The solution should begin to diffuse into the cubes turning the cubes pink staring on the outside and working its way in as it diffuses. Lab 4: Diffusion and Osmosis Experimental Design Procedure: 4. Remove the cubes and cut them in half: 5. Measure the size of the portion that the NaOH solution did not reach (the white part or so-called “final volume”) using a standard mm ruler: 6. Calculate the percent Volume of the cube that the solution diffused into… Lab 4: Diffusion and Osmosis Experimental Design Procedure: 4. Remove the cubes and cut them in half: 5. Measure the size of the portion that the NaOH solution did not reach (the white part or so-called “final volume”) using a standard mm ruler: 6. Calculate the percent Volume of the cube that the solution diffused into… Lab 4: Diffusion and Osmosis Experimental Design Procedure: 7. Obviously good science does not consist of one trial…Explain what you should do and what should be done with the data. Many trials should be performed for each cube. In addition, the mean, standard deviation and standard errors should be calculated for each cube as well and then a bar graph should be generated with standard errors as the error bars: Trial # Percent Volume of diffusion 2cm3 block (%) Percent Volume of diffusion 1cm3 block (%) Percent Volume of diffusion 0.5cm3 block (%) 1 36 50 100 2 38 55 98 3 41 51 94 4 34 54 100 5 36 60 99 Mean +/- SD 37 +/- 2.6 58 +/- 3.9 98 +/- 2.5 Standard Error 1.2 1.8 1.1 100 90 80 70 60 50 40 30 1 2 3 How else can we display this data? Lab 4: Diffusion and Osmosis Experimental Design Procedure: 8. Another excellent representation of the data (X-Y Scatter Plat): Percent diffused 100 90 80 70 60 50 40 30 0 0.5 1 1.5 Surface Area (cm3) 2 2.5 Lab 4: Diffusion and Osmosis Experimental Design What must be controlled? 1. Time the cube is inside the NaOH solution 2. Temperature of the solutions (remember that the higher the temp, the greater the diffusion rate) 3. Same amount of NaOH solution in each beaker 4. The size of the agar blocks, etc… Lab 4: Diffusion and Osmosis Answer these questions 1. It must be isotonic to the cytoplasm of your cells. You can also say it must have the same water potential as your cells. Since our cells are flaccid, Ψp=0 and therefore Ψs or the IV solution must match Ψs of the cytosol of your cells. 2. Pure water has a Ψs=0 and therefore a Ψ=0 since Ψp=0. Therefore the water potential is higher than that of the cytosol of your cells and water will net flow in causing lysis. Your blood will become hypotonic relative to the cytoplasm of your cells. 3. Determine the osmolarity (solute concentration) of their blood and match it. Lab 4: Diffusion and Osmosis Lab 4: Diffusion and Osmosis Answer these questions Lab 4: Diffusion and Osmosis Lab 4: Diffusion and Osmosis Pair 1 Pair 2 Pair 3 Pair 4 Control Inside Cell 1M Sucrose 1M NaCl 1M glucose 1M NaCl Water Outside Cell 1M Glucose 5% Ovalbumi n 1M NaCl 1M sucrose Water Lab 4: Diffusion and Osmosis Inside Cell Pair 1 Pair 2 Pair 3 Pair 4 Control 1M Sucrose 1M NaCl 1M glucose 1M NaCl Water 1M Glucose 5% Ovalbumi n 1M NaCl 1M sucrose Water Initial Mass (g) Outside Cell Final Mass after 30 Lab 4: Diffusion and Osmosis Inside Cell Pair 1 Pair 2 Pair 3 Pair 4 1M Sucrose 1M NaCl 1M glucose 1M NaCl 1M Glucose 5% Ovalbumin 1M NaCl Control Water Initial Mass (g) Outside Cell Final Mass (g) after 30 min Percent Change 1M sucrose Water Lab 4: Diffusion and Osmosis Pair 1 Pair 2 Pair 3 Pair 4 Control Inside Cell 1M Sucrose 1M NaCl 1M glucose 1M NaCl Water Outside Cell 1M Glucose 5% Ovalbumi n 1M NaCl 1M sucrose Water Lab 4: Diffusion and Osmosis Lab 4: Diffusion and Osmosis Lab 4: Diffusion and Osmosis Elodea Aquatic Plant (not algae) Lab 4: Diffusion and Osmosis Plasmolysis Lab 4: Diffusion and Osmosis Design an experiment to determine the water potential of potato cells