Chemical Formulas and Naming - Lighthouse Christian Academy
advertisement

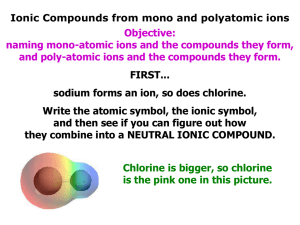
2.6 Ionic Compounds: Chemical Formulas and Naming (7.3 pg 183-191) • A chemical formula represents the relative proportion of elements in a compound. • There are 3 general types of ionic compounds that we need to know how to name – they all follow the same basic rule: the total ion charge for the compound is equal to 0. • The three types are: – Binary ionic compounds = compounds that only have two elemental ions bonded together (‘binary’ = 2; Table 1 p.183). E.g. (NaCl) or (CaF2) • The three types are: – Multivalent ionic compounds = compounds containing elements that can form more than one ion – the element’s ions can have different charges – E.g. (Iron can be Fe2+ or Fe3+). • The three types are: – Polyatomic ionic compounds = compounds that have ‘group ions’ as either the anion or the cation – e.g. (SO42-). See Table 4 p.189 and your Data Pages. • LET’S LOOK AT EACH TYPE OF IONIC COMPOUND INDIVIDUALLY! • WE’RE GOING TO NEED OUR TEXTBOOKS FOR THIS – OPEN ‘EM UP TO CYU 7.3 PG.191 • GREAT INTRODUCTORY VIDEO: • http://www.youtube.com/watch?v=URc75hoKGLY • Binary ionic compounds; some examples include Al2S3, CaF2, NaCl, etc. – Naming binary ions is the easiest. First name the cation (always listed first) then write the name of the anion (always written second). Finally, change the end of the anion to –ide. – For example Al2S3 = Aluminum Sulfide • Let’s try to name the other two examples listed above!!! • Binary ionic compounds; some examples include Al2S3, CaF2, NaCl, etc. – There are 2 ways to determine the formula of binary ionic compounds (see pg 184-186). – Here’s one way: look up the anion and the cation and write them down with their charges. (for this example, we’re determining the formula for Germanium Oxide) – Ge4+ O2– Then (if necessary) multiply the ion charges on the elements by whatever number it takes to get the charges to balance. Here we’re using the ‘criss-cross’ or ‘drop & swap’ method. – Ge4+ O2- becomes Ge2O4 and since both #’s can be divided by 2 we reduce it to GeO2 • Multivalent ionic compounds; some examples include CuCl2, Cu2O, Fe2O3 – Naming multivalent compounds involves the same steps as binary compounds. First name the cation, then write down the charge on the cation with roman numerals in brackets. Then name the anion but change the ending to –ide. To figure out the charge on the cation you will need to use the charge from the anion. – To name SnO2: – 1st figure out the Charge on Sn – Now name the cation and anion : Tin (IV) oxide • Multivalent ionic compounds; some examples include CuCl2, Cu2O, Fe2O3 – Drawing the formula for multivalent is the same as with binaries. Start by writing the symbols with their charges. Then use the criss-cross method. • GREAT VIDEO: http://www.youtube.com/watch?v=p9iQ5Qn42DM • Polyatomic Ionic compounds include either a cation or anion that contains multiple elements (‘polyatomic’ = multiple atoms). Some examples include Al2(SO4)3, (NH4)2O – Naming polyatomic ions is the same as naming binary ionic compounds. First name the cation then name the anion. – For example Al2(SO4)3 is named aluminum sulfate. You will need to refer to the list of polyatomic ions in your Data Pages to determine the names/charges. • Polyatomic Ionic compounds include either a cation or anion that contains multiple elements (‘polyatomic’ = multiple atoms). Some examples include Al2(SO4)3, (NH4)2O – To determine the formula from the name, write the symbol for the cation first and then for the anion. Then write their charges. If there will be more than 1 polyatomic ion, it has to be placed in brackets. – Then use the criss-cross method. – Remember to treat the polyatomic ion as a single unit. • THAT’S ALL FOR IONIC COMPOUNDS.....FOR NOW