Density

Everyone Ever Tell You are Dense?
Well, you all are…
Because you all have these two properties
You are made up of
MATTER …which means you have mass
You all have
VOLUME …which means you take up space
When You Combine Mass and Volume, you get…
Density
A measure of the amount of matter that occupies a given amount of space
SPACE
Amount of Matter
Density
Aim: How is density determined?
What is Density?
• Which one is more dense, a bowling ball or a soccer ball?
• Even though they are both approximately the same size and shape, the bowling ball is much more dense.
Density
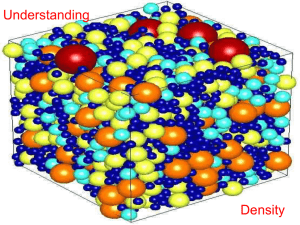
• Therefore, density depends on the mass of particles and how closely packed they are.
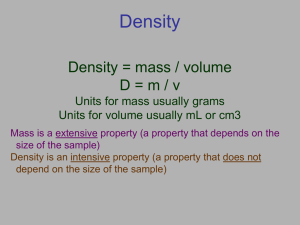
• Density = Mass
Volume
Let’s put it all together…
• DENSITY is the ratio of the mass of a substance to the volume of the substance
UNITS: commonly expressed as grams per cubic centimeter for solids and
liquids and grams per liter for gases
So now you’re going to say we need more units, right?
Right!!!!
Units for Density =
Grams per cubic centimeter g/cm 3
What Do All Those Letters Mean?
Quick Visual Aid
Want to See An Example Question?
An unknown object has a mass of 15 grams and a volume of 5 cm 3 . What is the density of this object?
Density = Mass ÷ Volume
Density = 15 grams ÷ 5 cm 3
Density = 3.0 g/cm 3
Your Turn!!!
An unknown liquid has a volume of 6 cm3 and a mass of
6 grams. What is the density of this liquid?
Density = Mass ÷ Volume
Density = 6 grams ÷ 6 cm 3
Density = 1.0 g/cm 3
Find the Density of the Cube
Mass = 480 grams
480 g
=
2 g/cm 3
240 cm 3
10 cm
3 cm
8 cm
Density Problems
• Calculate the density of a rock that has a mass of 40 grams and a volume of 5 cm 3 .
• 40g/5 cm 3 = 8 g/cm 3
• An unknown sample has a density of 8 g/cm 3 . If the mass of the sample is 16 grams, what is its volume?
• 8 g/cm 3 = 16g/V = 2 cm 3
• An unknown sample has a density of 10 g/cm 3 and a volume of
2 ml. What is the mass of the sample?
• 10 = M/2 ml = 20 g
Density Facts
• The Density of a substance remains the same regardless of size, shape, or mass of sample!!!
• Example: The density of a piece of aluminum is 2.7 g/cm 3 .
• If you cut the Al bar in half, the density of each piece is still 2.7 g/cm 3
• Even if you roll the aluminum into a sheet the density will still remain the same
Density of Aluminum
D = 2.7 g/cm3 D = 2.7 g/cm 3
By simply cutting the bar in half, the density remains the same!!!
What will change the density of an object?
• Temperature
• As the temperature of a substance INCREASES the density DECREASES due to the expansion of the substance
Inverse Relationship
Most substances EXPAND when heated and CONTRACT when cooled
• Warm air rises because it is LESS dense
• Cold air sinks because it is MORE dense
What will change the density of an object?
• PRESSURE
• As pressure on a substance INCREASES the density
INCREASES
DIRECT RELATIONSHIP
• Material DENSER than water will SINK in water
• Material LESS DENSE than water will FLOAT
• Materials with a density EQUAL TO water may remain at ANY
LEVEL
• When several objects of different densities float on water, the LEAST DENSE objects float HIGHEST.
Water:
Object D:
Object C:
Object B:
Object A:
1 g/ml
2 g/cc
1 g/cc
0.4g/cc
0.8g/cc
A
B
C
D
D
C
A
B
Volume (cc)