Understanding Density
advertisement

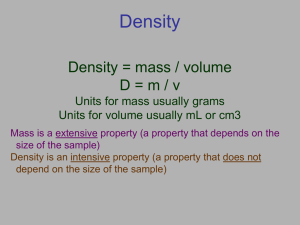
Understanding Density What is Density? • The ratio of the mass of a substance to its volume M D= V – Volume – amount of space an object takes up – Mass – the amount of matter (stuff) in an object What is Density? – Volume – amount of space an object takes up – Mass – the amount of matter (stuff) in an object – So density is the amount of stuff in a certain space…. – The more stuff in a space the higher the density • Which box has more stuff in it? • Which box has the greatest density? • Why does this box have the greatest density? •BECAUSE IT HAS MORE STUFF IN IT!!! Think about it… • What is the most dense “phase” of matter? – SOLID….why? • Has the most “stuff” (matter) in the smallest space • Huge Balloon versus Golf Ball. Which on is more dense? • Golf Ball …. Why? • Has the most “stuff” (matter) in the smallest space Heating an object… Therefore: same “stuff” (matter) in a bigger space = lower density Expands- volume increases = more space Matter – Stuff inside remains the same Temperature & Density volume density Temperature & Volume temperature temperature Breaking up … • If you break an object of uniform composition in half, what happens to its density? MASS = 100 g VOLUME = 10 cm3 DENSITY = 10 g/ cm3 Breaking up … • If you break an object of uniform composition in half, what happens to its density? MASS = 50 g VOLUME = 5 cm3 DENSITY = 10 g/ cm3 MASS = 50 g VOLUME = 5 cm3 DENSITY = 10 g/ cm3 WHY – MASS AND VOLUME CHANGE PROPORTIONALLY (EQUALLY) Breaking up … • If you break an object of uniform composition in half, what happens to its density? MASS = 50 g VOLUME = 5 cm3 DENSITY = 10 g/ cm3 MASS = 25 g VOLUME = 2.5 cm3 DENSITY = 10 g/ cm3