Thermochemistry II
advertisement
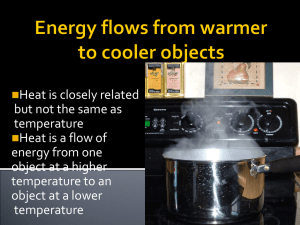
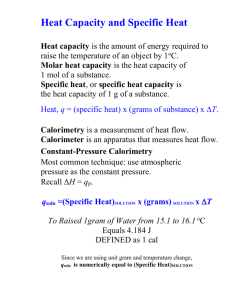
Thermochemistry II Heat capacity and Calorimetry Calorie A calorie is a unit of heat. It is defined as the quantity of heat (q) needed to raise one gram of pure water one degree C. calorie versus Calorie Dietary units of energy are Calories. 1000 calories = 1 kilocalorie = 1 Calorie Joules Joule is the SI unit for energy. 1 joule= 0.2390 cal 1 cal = 4.184 joule Heat Capacity The amount of heat needed to increase the temperature of an object exactly 1 degree C is the heat capacity of an object. Different objects have different heat capacities. Which heats faster, Cu or Al? Heat Capacity Specific heat capacity Specific heat capacity or Specific Heat, is the amount of heat it takes to heat 1 gram of a substance 1 degree C. Examples If a chunk of silver has a heat capacity of 42.8 J/°C. If the silver has a mass of 181 grams, calculate the specific heat of silver. Answer: 0.236 J/(g °C) Calorimetry Enthalpy ∆H is the heat under constant pressure. What pressure is constant? Atmospheric Pressure. q= ∆H = m x C x ∆T ∆H is the heat under constant pressure. C is the heat capacity. In calorimetry, C is the heat capacity of water, the item heated.(system or surrounding?) ∆T is the change in temperature. T final – T initial = ∆T Bomb Calorimetry ∆H versus q In a bomb calorimeter, the pressure is not constant. With combustion under a constant volume, pressure will increase. Therefore we must use q and not ∆H. Example: Go to worksheet.