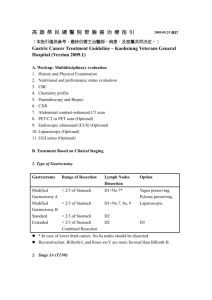
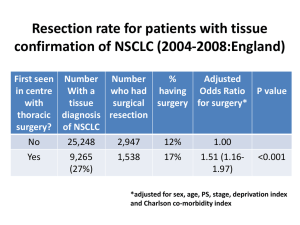
Diagnosis & Surgical Management of Gastric Malignancies
advertisement

Diagnosis & Surgical Management of Gastric Malignancies PETER J. DIPASCO, MD ASSISTANT PROFESSOR OF SURGERY DEPARTMENT OF SURGERY – SECTION OF SURGICAL ONCOLOGY THE UNIVERSITY OF KANSAS MEDICAL CENTER FRIDAY, APRIL 4TH, 2014 ACOS GENERAL SURGERY IN-DEPTH REVIEW Disclosure I have no disclosures Epidemiology Third leading cause of cancer death worldwide Overall declining Endemic areas persist Refrigeration Histologic pattern is shifting from predominantly intestinal type (distal) to diffuse type (proximal / cardia) Factors Increasing or Decreasing Gastric CA Increase risk Family history Diet (high in nitrates, salt, fat) Familial polyposis Gastric adenomas Hereditary nonpolyposis colorectal cancer Helicobacter pylori infection Atrophic gastritis, intestinal metaplasia, dysplasia Previous gastrectomy or gastrojejunostomy (>10 y ago) Tobacco use Ménétrier’s disease Decrease risk Aspirin Diet (high fresh fruit and vegetable intake) Vitamin C Gastric Cancer Work-up/Staging Standard CT chest, abdomen/pelvis PET-CT Endoscopic Ultrasound Controversial Laparoscopy Peritoneal washing Gastric Cancer – Surgical Controversies Resection Margins Extent of Lymphadenectomy Role of Sentinel Lymph Node Biopsy Minimally-Invasive Resection Endoscopic Mucosal Resection (EMR) Laparoscopic Resection Surgical Margins Total vs. Subtotal Gastrectomy? Goals Oncologically-Sound Resection 5 - 6 cm gross margins ideal • minimal 2-3 cm margins En-bloc resection if necessary • partial pancreas, partial colon, spleen, etc. Low Morbidity Avoid (if possible): • total gastrectomy • injury to the distal common bile duct Surgical Margins Subtotal vs. Total Gastrectomy? Factors Influencing Operation Extent of disease Histological type – total gastrectomy Intestinal – potentially subtotal gastrectomy Diffuse Location (for intestinal type) • • • • Lower – subtotal gastrectomy Mid – near-total gastrectomy Upper – total gastrectomy < 2 cm of GE junction- Esophagogastrectomy D1 vs. D2 Resection – Where do we stand? Definitions Theoretical Considerations Review of Clinical Trials Controversy Japanese vs. Western Data Proposed Approaches Conventional Utilizing the Maruyama Index Lymph Node Stations (Japanese) Synopsis of Definitions - D1 vs. D2 D1 Lymphadenectomy Lymph nodes directly adjacent gastric wall & 2 – paracardial 3 & 4 – lesser and greater curvature 5 & 6 – peri-pyloric 1 Synopsis of Definitions – D1 vs. D2 D2 Lymphadenectomy (“Radical Lymphadenectomy”) Additional tissue (en bloc): Greater and lesser omentum Superior leaf of mesocolon Pancreatic capsule Lymph nodes: Infra/supraduodenal areas Hepatic and common hepatic arteries Celiac artery Splenic artery Organs Distal pancreatectomy (station 11 lymph nodes) Splenectomy (station 10 lymph nodes Radical Lymphadenectomy (D2) Theoretical Considerations Pros More Accurate Staging (Prognostic Information) Lymph node status likely to influence adjuvant therapy Better Locoregional Control More extensive surgery Removes occult nodal disease Improved Survival Retrospective Japanese data No Excess Morbidity/Mortality Japanese experience Radical Lymphadenectomy (D2) Theoretical Considerations Cons Advanced disease not amenable to more radical locoregional surgery No “true” survival advantage Survival advantage of radical surgery merely an artifact of more accurate staging by nodal clearance “Stage migration” Western data does not support Japanese experience Excess morbidity/mortality/cost Western data Minimally Invasive Resection Types Laparoscopic Intraperitoneal wedge resection distal gastrectomy Intragastric Endoscopic Mucosal Resection (EMR) Indication Intramucosal lesion Low-risk of lymph node involvement Endoscopic Mucosal Resection Selection Criteria Histology/Differentiation Well and/or moderately differentiated adenocarcinoma Or papillary adenocarcinoma Confined to the mucosa Without evidence of venous or lymphatic involvement Size Less than 2 cm if type IIA (superficially elevated) Less than 1 cm if type IIB or IIC (superficially depressed) Ulcer status None grossly on endoscopy None microscopically No clinical evidence of lymph node involvement Chemoradiation Therapy Adjuvant Chemoradiation Therapy Landmark Intergroup 0116 Trial 556 randomized patients Vs. Surgery Alone 5-FU based regimen with concurrent XRT Improvement: Locoregional recurrence Median survival Overall survival Standard of care for stage IB and higher Chemoradiation Therapy Neoadjuvant Chemotherapy MAGIC Trial 503 randomized patients Vs. Surgery Alone epirubicin, cisplatin, continuous 5-FU Stage II or greater non-metastatic disease Post-op chemotherapy Improvements: Progression-free survival Overall survival Neoadjuvant chemoradiation Therapy Ongoing Studies Currently useful in borderline resectable patients Summary Performance of oncologically-sound, low-morbid gastric resection & reconstruction Avoid total gastrectomy and achieve microscopic (-) margins Future Trends (early cancer) Minimally-invasive resections Endoscopic mucosal resections Role of “radical lymph node dissection” (D2) still controversial in Western countries Avoid splenectomy and/or pancreatectomy Future trends Use of Maruyama Index (MI) Role for palliative resection for symptomatic patients Important role for chemotherapy and radiation therapy CASE REPORT 58M recently admitted to OSH for abd pain and early satiety. Other complaints include post prandial pain in mid-epigastrium and a feeling of food getting stuck. EGD showed proximal gastric cancer. Diagnostic Tests? Imaging? Staging? Surgical Plan?