Snímek 1
advertisement
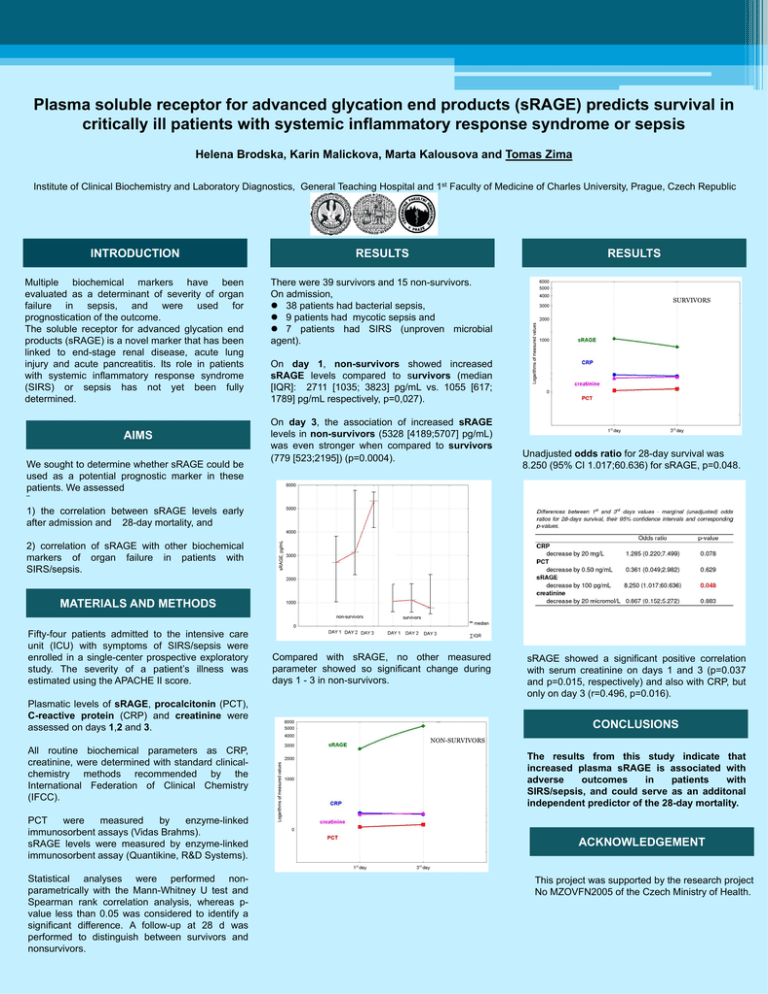
Plasma soluble receptor for advanced glycation end products (sRAGE) predicts survival in critically ill patients with systemic inflammatory response syndrome or sepsis Helena Brodska, Karin Malickova, Marta Kalousova and Tomas Zima Institute of Clinical Biochemistry and Laboratory Diagnostics, General Teaching Hospital and 1st Faculty of Medicine of Charles University, Prague, Czech Republic INTRODUCTION RESULTS Multiple biochemical markers have been evaluated as a determinant of severity of organ failure in sepsis, and were used for prognostication of the outcome. The soluble receptor for advanced glycation end products (sRAGE) is a novel marker that has been linked to end-stage renal disease, acute lung injury and acute pancreatitis. Its role in patients with systemic inflammatory response syndrome (SIRS) or sepsis has not yet been fully determined. There were 39 survivors and 15 non-survivors. On admission, 38 patients had bacterial sepsis, 9 patients had mycotic sepsis and 7 patients had SIRS (unproven microbial agent). AIMS We sought to determine whether sRAGE could be used as a potential prognostic marker in these patients. We assessed ¨ 1) the correlation between sRAGE levels early after admission and 28-day mortality, and RESULTS SURVIVORS On day 1, non-survivors showed increased sRAGE levels compared to survivors (median [IQR]: 2711 [1035; 3823] pg/mL vs. 1055 [617; 1789] pg/mL respectively, p=0,027). On day 3, the association of increased sRAGE levels in non-survivors (5328 [4189;5707] pg/mL) was even stronger when compared to survivors (779 [523;2195]) (p=0.0004). Unadjusted odds ratio for 28-day survival was 8.250 (95% CI 1.017;60.636) for sRAGE, p=0.048. 2) correlation of sRAGE with other biochemical markers of organ failure in patients with SIRS/sepsis. MATERIALS AND METHODS Fifty-four patients admitted to the intensive care unit (ICU) with symptoms of SIRS/sepsis were enrolled in a single-center prospective exploratory study. The severity of a patient’s illness was estimated using the APACHE II score. Compared with sRAGE, no other measured parameter showed so significant change during days 1 - 3 in non-survivors. Plasmatic levels of sRAGE, procalcitonin (PCT), C-reactive protein (CRP) and creatinine were assessed on days 1,2 and 3. sRAGE showed a significant positive correlation with serum creatinine on days 1 and 3 (p=0.037 and p=0.015, respectively) and also with CRP, but only on day 3 (r=0.496, p=0.016). CONCLUSIONS NON-SURVIVORS All routine biochemical parameters as CRP, creatinine, were determined with standard clinicalchemistry methods recommended by the International Federation of Clinical Chemistry (IFCC). PCT were measured by enzyme-linked immunosorbent assays (Vidas Brahms). sRAGE levels were measured by enzyme-linked immunosorbent assay (Quantikine, R&D Systems). Statistical analyses were performed nonparametrically with the Mann-Whitney U test and Spearman rank correlation analysis, whereas pvalue less than 0.05 was considered to identify a significant difference. A follow-up at 28 d was performed to distinguish between survivors and nonsurvivors. The results from this study indicate that increased plasma sRAGE is associated with adverse outcomes in patients with SIRS/sepsis, and could serve as an additonal independent predictor of the 28-day mortality. ACKNOWLEDGEMENT This project was supported by the research project No MZOVFN2005 of the Czech Ministry of Health.











