Neuropathic Pain
advertisement

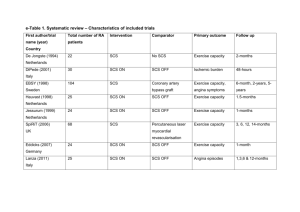
Spinal Cord Stimulation for Pain Alon Y. Mogilner, MD, PhD Associate Professor of Neurosurgery Director, Center for Neuromodulation NYU Langone Medical Center Disclosures • Medtronic neurological: – Consultant, fellowship/grant support • St. Jude Medical: – Grant support, consultant • Boston Scientific – Grant Support Pain: Anatomy and Physiology • Nociceptive pain: – Pain arising from a result of tissue damage and concomitant activation of nociceptors (sensory receptors) in the peripheral nervous system • • • • Trauma Inflammation Visceral distention Neoplastic infiltration – Described as “sharp, aching, throbbing” • Neuropathic pain: – Pain arising from a lesion or dysfunction in the peripheral or central nervous system • • • • Radiculopathy Postherpetic neuralgia Peripheral neuropathy Central (post-stroke) pain – Described as “burning, electrical, tingling, shooting” Neuromodulation • Generally used for neuropathic pain – Dysesthesias- Unfamiliar, unpleasant sensations often burning or electrical – Paresthesias- paroxysmal, shooting, stabbing – Allodynia- mild stimuli are perceived as painful Neuropathic Pain Sustaining Mechanisms – Primary afferent or CNS nociceptive hyperactivity – loss of central inhibitory mechanisms – increased sympathetic efferents Gate theory of pain • Introduced by Melzack and Wall • Stimulation of large myelinated Aß fibers results in paresthesias that block the activity of small A∂ (thinly myelinated), and C (nonmyelinated) fibers. History of spinal cord stimulation • Spinal cord stimulation (SCS) was first used in the late 1960s and 1970s. • The first fully implantable system was available in the early 80s. • It is most commonly used in the US for the treatment of Complex regional pain syndrome (CRPS) or Failed Back surgery syndrome (FBSS), but is more often used in Europe for treatment of peripheral vascular disease and non-operative angina. How does it work? • SCS is thought to activate paininhibiting neuronal circuits within the dorsal horn and inducing paresthesias. Modified Gate Control Effects on neurotransmitters SP content in human CSF also appears to increase as a result of therapeutic SCS GABA and glycine increase in DH of rats with SCS; GABA-B antagonists counteract the effects of SCS. Supraspinal effects • The activation of supraspinal circuits is also evident from a growing number of studies – microdialysis studies on transmitter release in the PAG of the rat – positron emission tomography in angina patients submitted to SCS – changes in c-fos and stress proteins in the rat with experimental SCS Other forms of stimulation • All thought to involve gate theory • Local effects based on area being stimulated. • With upstream or downstream effects, based on location. • Neuromodulatory device usually placed proximally to region suspected to be aberrantly firing. SCS: General Overview • Indications: – Neuropathic pain syndromes • Failed Back Surgery Syndrome (FBSS) – Most common indication in US for SCS – Aka Post laminectomy syndrome – Persistent pain in the back and legs despite spinal surgery • Complex Regional Pain Syndrome (CRPS, RSD) • Neuropathies of varying etiologies – Diabetes, Vascular disease, HIV, idiopathic, neoplastic • Ischemic disease – More commonly used in Europe » Angina » PVD SCS: Patient Selection • Most common clinical indications: – Post laminectomy syndrome, aka “failed back surgery syndrome” (FBSS) • Persistent pain in the back and legs despite spinal surgery – Complex regional pain syndrome (CRPS), aka RSD (Reflex sympathetic dystrophy) of the limbs – Angina Pectoris – Ischemic pain of the limbs • • • • • So which patients may benefit from neuromodulation? (Based on SCS data) 1) pain not associated with malignancy; 2) poor response to conservative treatment for at least 6 months 3) remedial surgery inadvisable 4) no major psychiatric disorder, including somatization complaints • 5) willingness to stop inappropriate drug use before implantation • 6) no secondary gain or litigation involved • 7) ability to give informed consent for the procedure. The supporting literature for patient selection for spinal cord stimulation • The clinical factors that were found to be reliable indicators for a good response to SCS in FBSS at 10 year follow up were: – 1) early treatment (0–3 years) after first failed back surgery; – 2) predominance of neuropathic leg pain; – 3) absence of psychological conditions, such as untreated depression. The role of psychological testing • Psychological testing allows for: – evaluation and treatment of comorbid psychiatric conditions – assessment of overall mechanisms and support systems for dealing with pain. • One study revealed that the predictive value of psychological testing correlated significantly with short-term follow-up findings only. • May involve Oswestry Disability Index, the McGill Pain Questionnaire (short form), the Roland Morris Questionnaire, and the Beck Depression Inventory. Predictive value of psychological testing • Many studies have examined the value of psychological testing in predicting success with SCS – Daniel et al calculated an 80% accuracy rate using the MMPI and BDI for predicting success. – Burchiel et al. found that the BDI score and mania scale on the MMPI emerged as predictors. Less helpful in a subsequent study. – Long et al reported a 33% success rate in unscreened patients compared with 70% in screened patients. The trial phase • Varies from site to site • After selection, patients then undergo a trial period of stimulation for 2 to 7 days to assess their response to the treatment. – Patients are often asked to record their VAS in a diary multiple times – Individuals who achieve >50% or greater pain relief are candidates for internalization of the device Neurostimulation Trial Goals • The trial provides an opportunity to measure the effectiveness of neurostimulation without making a long-term commitment: – Gauge patient response – Provides an adjustment period – Identify therapy parameters • The goal is at least a 50% reduction in pain without intolerable side effects – Patient-specific goals may include less pain reduction but improved quality of life Percutaneous Trial • Most common form of trial • Performed by: – “Pain Management” » Anesthesiologists » Physiatrists » Neurologists – Neurosurgeons – Orthopedic Surgeons • Should be performed for trial unless there are contraindications Percutaneous SCS • Advantages: – Ease of placement • Does not require laminotomy – Decreased pain – Can screen multiple spinal levels – Can be placed by non-surgeons • Disadvantages: – Cannot always be accomplished (scar tissue, fusion mass) – Higher current requirements – Can be placed by non-surgeons – Neurosurgeons not traditionally trained in epidural needle placement Percutaneous leads SCS Leads – surgical and percutaneous Needle placement • Thoracic cord stimulation – Back and LE • Entry point in mid-upper lumbar spine • Tip of lead: T8 • Foot pain: – Tip of lead T12 – Entry point lower in lumbar spine • Cervical Stimulation – Entry point in mid-upper thoracic spine – Lead tip in cervical spine Percutaneous Lead Placement Intraoperative Test Stimulation A screening system is used during intraoperative test stimulation: > Includes an external power source connected to an implanted lead or percutaneous extension. > Is used to identify lead location. > Is used to identify the stimulation parameters – amplitude, pulse width, and rate – that produce the paresthesia to provide adequate pain relief. Complications • Trial complications – – – – CSF leak Increased pain Hematoma Infection – – – – All of the above, plus… Lead migration Infection Malfunction • Permanent complications Permanent implant • Percutaneous or laminectomy – Percutaneous • Advantages: – Easy to perform – Less invasive • Disadvantages: – Higher risk of lead migration – Higher current requirements – Laminectomy • Advantages: – Low risk of lead migration – Lower current requirements – “Better” qualitative sensation • Disadvantages: – Requires a surgeon – More invasive Trial to permanent SCS: Results SCS: Summary • Better than best medical therapy and repeat surgery (class I evidence) in cases of: – Postlaminectomy syndrome • Effective in cases of: – Complex regional pain syndrome (RSD) – Peripheral neuropathic pain – Ischemic pain of extremities Thank you