Abraxane CTOS 2011
advertisement

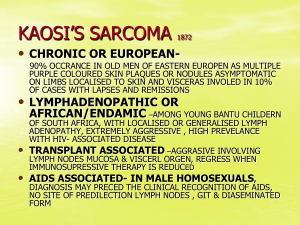
A PHASE II STUDY OF NANOPARTICLE ALBUMINBOUND (NAB) PACLITAXEL IN THE TREATMENT OF PATIENTS WITH UNRESECTABLE OR METASTATIC SARCOMA James E. Butrynski1, Rangarajan Nadadur2, Thierry Jahan3, Victoria Chua4, Claire Bautista Lazaro4, Sant Chawla4 1. Dana-Farber Cancer Institute, Boston, MA USA 2. Massachusetts Institute of Technology, Boston, MA USA 3. University of California at San Francisco, CA USA 4. Sarcoma Center, Santa Monica, CA USA INTRODUCTION •nab-Paclitaxel is a novel biologically interactive, albuminbound Paclitaxel combining a protein with a chemotherapeutic agent in a particle form. In principle, this composition provides a novel approach of increasing intra-tumoral concentration of the drug by a receptormediated transport process allowing transcytosis across the endothelial cell wall. This albumin-specific receptor mediated process involves the binding of a specific receptor (gp60) on the endothelial cell wall, resulting in activation of a protein caveolin-1, which initiates an opening in the endothelial wall with formation of caveolae, with transport of the albumin bound chemotherapeutic complex via these caveolae to the underlying tumor interstitium. A protein specifically secreted by the tumor (SPARC) binds and entraps the albumin, allowing release of the hydrophobic drug to the tumor cell membrane. Transport of nab-Paclitaxel via this gp-60/caveolin1/caveolae/SPARC pathway potentially increases intratumoral concentration of drug while reducing toxicity to RESULTS (2) TOXCITY •3 deaths on trial all due to disease progression •grade 4 neutropenia (2) •grade 3 neutropenia (3) •grade 3 anemia (1) •grade 3 hypocalcemia (1) •grade 3 hyponatremia (1) •grade 3 hyperglycemia (1) RESPONSE 15 assessable patients •Stable Disease (SD) 3 subtypes included ESS, LMS and EMC. •ESS patient achieved “clinical benefit” as defined in this trial. •LMS and EMC had SD of duration less than 6 months •Progressive Disease (PD) 12. normal tissue. •One patient withdrew consent after one cycle. •There is a need to identify new active agents with a high probability to target the tumor to improve overall survival for sarcoma patients. •No patients with angiosarcoma or other vascular tumor subtypes were entered on trial. •The goal of this trial is to explore the activity of nabPaclitaxel for patients with sarcoma. OBJECTIVES PRIMARY OBJECTIVE •To determine the clinical benefit rate by modified RECIST (CR, PR or SD for 6 months) of nab-Paclitaxel in patients with unresectable or metastatic soft tissue or bone sarcoma SECONDARY OBJECTIVES •To assess safety and tolerability of nab-Paclitaxel •To assess duration of response METHODS •This single center (Sarcoma Center, Santa Monica, CA USA) study utilized a Simon two-stage design. •No prior paclitaxel was allowed. •Eligible unresectable or metastatic sarcoma patients who underwent informed consent were treated with nab Paclitaxel 150mg/m2 IV on day 1,8,15 every 28 days. •Tumor assessments every 2 cycles •All patients who receive at least one dose of nabpaclitaxel were evaluable for response and toxicity •Toxicity was monitored throughout treatment RESULTS (1) •19 patients were enrolled and treated. PATIENT CHARACTERISTICS •M:F 12:7 •ECOG PS 0:1 1:18 •median age 48 years (range 26-81 years) •Median number of prior regimens 4 (range 1-8) DIAGNOSES ENROLLED •Malignant Fibrous Histiocytoma (MFH) (4) •Leiomyosarcoma (LMS) (3) •Osteosarcoma (OS) (3) •Extraskeletal Myxoid Chondosarcoma (EMC) (2) •Synovial Sarcoma (SS) (2) •Endometrial Stromal Sarcoma (ESS) (1) •Peripheral Neuroectodermal Tumor (PNET) (1) •Chondrosarcoma (CS) (1) •Myxoid Liposarcoma (MLS) (1) •Pleomorphic Sarcoma (PS) (1) CONCLUSIONS •Nab-Paclitaxel has some activity in ESS, LMS, EMC •Acceptable safety and tolerability •Further study in ESS, LMS, EMC is warranted •Further study in sarcomas of the vascular tumor subtype (Angiosarcoma, Epithelioid hemangioendothelioma) should be considered FUTURE DIRECTION •Clinical studies have shown that upregulation of both caveolin-1 and SPARC (secreted protein, acidic, rich in cysteine) are associated with progression of cancer Interstitial accumulation of albumin is facilitated by SPARC. Hawkins and colleagues have reported that tumor SPARC levels were highly associated with treatment responses in patients with SCCHN receiving nab-Paclitaxel •Assesment of SPARC protein expression from archival tumor tissue and correlate to response REFERENCES •Desai N, et al (2006). Increased Antitumor Activity, Intratumor Paclitaxel Concentrations and Endothelial Cell Transport of Cremophor-Free, Albumin-Bound Paclitaxel, ABI-007 Compared with Cremophor-Based Paclitaxel. Clin Cancer Res; 12(4): 1317-1324 •Olawaiye AB,et al (2008) Epithelioid Angiosarcoma of the Uterus: A Review of Management. Arch Gynecol Obstet 278(5): 401-404 •Hawkins, M, et al. Experience with ABI-007 administered by intra-arterial infusion to patients with cancer of the head and neck. In: 23rd Annual Chemotherapy Foundation Symposium, 2005, pp. Abstract #25 ACKNOWLEDGEMENTS The authors wish to acknowledge the patients and families who participated in this study and to Abraxis for financial support for this study.