Features of Epstein Barr Virus (EBV) reactivation after reduced intensity conditioning (RIC)
unrelated umbilical cord blood transplantation (UCBT)
Z Peric,1,2 X Cahu, 1 P Chevallier,1 E Brissot, 1 T Guillaume,1 J Delaunay,1 S Ayari,1 V Dubruille,1 S Le Gouill,1 B Mahe,1 T Gastinne,1 N Blin,1 B Saulquin,1 N Milpied,1 R Vrhovac, 2 JL Harousseau,1 P Moreau,1 M Coste- Burel,3 BM Imbert-Marcille,3 and M Mohty 1
1 CHU de Nantes, Hematology Department, Nantes, France 2 University Hospital, Hematology Department, Zagreb, Croatia 3 CHU de Nantes, Laboratoire de virology, Nantes, France
Introduction
Unrelated umbilical cord blood (UCB) is now being
increasingly used as an alternative stem cell source for
allogeneic stem cell transplantation (allo-SCT). Because of
the slow kinetics of immune reconstitution after UCBT,
previous studies showed that EBV reactivation and EBV
induced lymphoprolipherative disease (LPD) may be of
matter of concern.(1) However,more recent studies reported
an infectious-related mortality (IRM) incidence similar to that
of allo-SCT using HLA-matched unrelated donor. (2) This
single centre study assessed incidence and predictive factors
of EBV reactivation and LPD in 33 consecutive patients
undergoing RIC UCBT.
Patients and methods
In all, 33 consecutive patients who received a RIC UCBT for
hematological malignancies in a single institution (University
Hospital of Nantes) between January 2005 and June 2009
were included in this retrospective study. During the first six
months after allo-HSCT and in patients treated for GVHD, all
patients were weekly DNA-PCR screened in the peripheral
blood for EBV reactivation and were clinically monitored for
clinical features attributable to EBV. EBV viremia was
defined as 1000 copies of EBV DNA /105 cells. EBV LPD
was defined as biopsy- or autopsy proven posttransplantation lymphoma, or viremia along with
computerized tomography nodal or soft-tissue abnormalities
consistent with LPD. Patients with EBV viremia >1000
copies on at least two consecutive occasions were treated
with rituximab at a dose of 375 mg/m2 weekly until clearance
of EBV viremia (usually for a maximum of 4 infusions).
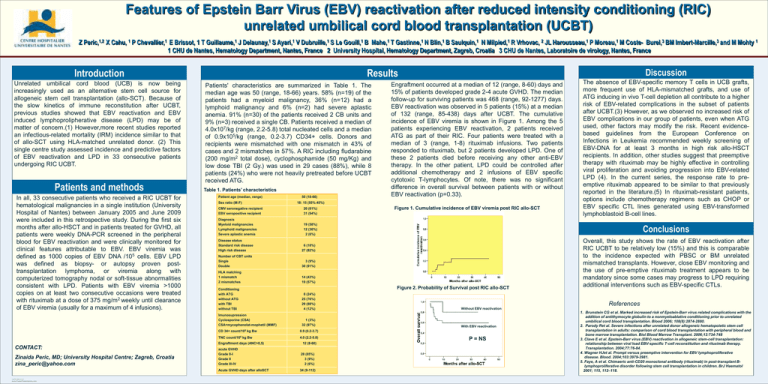
Patients' characteristics are summarized in Table 1. The
median age was 50 (range, 18-66) years. 58% (n=19) of the
patients had a myeloid malignancy, 36% (n=12) had a
lymphoid malignancy and 6% (n=2) had severe aplastic
anemia. 91% (n=30) of the patients received 2 CB units and
9% (n=3) received a single CB. Patients received a median of
4.0x107/kg (range, 2.2-5.8) total nucleated cells and a median
of 0.9x105/kg (range, 0.2-3.7) CD34+ cells. Donors and
recipients were mismatched with one mismatch in 43% of
cases and 2 mismatches in 57%. A RIC including fludarabine
(200 mg/m2 total dose), cyclophosphamide (50 mg/Kg) and
low dose TBI (2 Gy.) was used in 29 cases (88%), while 8
patients (24%) who were not heavily pretreated before UCBT
received ATG.
Table 1. Patients’ characteristics
Patient age (median, range)
Sex ratio (M:F)
CMV seronegative recipient
EBV seropositive recipient
20 (61%)
31 (94%)
Diagnosis
Myeloid malignancies
Lymphoid malignancies
Severe aplastic anemia
19 (58%)
12 (36%)
2 (6%)
Disease status
Standard risk disease
High risk disease
6 (18%)
27 (82%)
Number of CBT units
Single
Double
3 (9%)
30 (91%)
HLA matching
1 mismatch
2 mismatches
14 (43%)
19 (57%)
count/105
8 (24%)
25 (76%)
29 (88%)
4 (12%)
kg Bw
TNC count/106 kg Bw
Engraftment days (ANC>0,5)
POSTER TEMPLATE BY:
www.PosterPresentations.com
Figure 1. Cumulative incidence of EBV viremia post RIC allo-SCT
1 (3%)
32 (97%)
12 (8-60)
acute GVHD
Grade 0-I
Grade II
Grade III-IV
28 (85%)
3 (9%)
2 (6%)
Acute GVHD days after alloSCT
34 (9-112)
Overall, this study shows the rate of EBV reactivation after
RIC UCBT to be relatively low (15%) and this is comparable
to the incidence expected with PBSC or BM unrelated
mismatched transplants. However, close EBV monitoring and
the use of pre-emptive rituximab treatment appears to be
mandatory since some cases may progress to LPD requiring
additional interventions such as EBV-specific CTLs.
References
Without EBV reactivation
With EBV reactivation
0.9 (0.2-3.7)
4.0 (2.2-5.8)
The absence of EBV-specific memory T cells in UCB grafts,
more frequent use of HLA-mismatched grafts, and use of
ATG inducing in vivo T-cell depletion all contribute to a higher
risk of EBV-related complications in the subset of patients
after UCBT.(3) However, as we observed no increased risk of
EBV complications in our group of patients, even when ATG
used, other factors may modify the risk. Recent evidencebased guidelines from the European Conference on
Infections in Leukemia recommended weekly screening of
EBV-DNA for at least 3 months in high risk allo-HSCT
recipients. In addition, other studies suggest that preemptive
therapy with rituximab may be highly effective in controlling
viral proliferation and avoiding progression into EBV-related
LPD (4). In the current series, the response rate to preemptive rituximab appeared to be similar to that previously
reported in the literature.(5) In rituximab-resistant patients,
options include chemotherapy regimens such as CHOP or
EBV specific CTL lines generated using EBV-transformed
lymphoblastoid B-cell lines.
Conclusions
Figure 2. Probability of Survival post RIC allo-SCT
Conditioning
with ATG
without ATG
with TBI
without TBI
CD 34+
Zinaida Peric, MD; University Hospital Centre; Zagreb, Croatia
zina_peric@yahoo.com
50 (18-66)
Engraftment occurred at a median of 12 (range, 8-60) days and
15% of patients developed grade 2-4 acute GVHD. The median
follow-up for surviving patients was 468 (range, 92-1277) days.
EBV reactivation was observed in 5 patients (15%) at a median
of 132 (range, 85-438) days after UCBT. The cumulative
incidence of EBV viremia is shown in Figure 1. Among the 5
patients experiencing EBV reactivation, 2 patients received
ATG as part of their RIC. Four patients were treated with a
median of 3 (range, 1-8) rituximab infusions. Two patients
responded to rituximab, but 2 patients developed LPD. One of
these 2 patients died before receiving any other anti-EBV
therapy. In the other patient, LPD could be controlled after
additional chemotherapy and 2 infusions of EBV specific
cytotoxic T-lymphocytes. Of note, there was no significant
difference in overall survival between patients with or without
EBV reactivation (p=0.33).
18: 15 (55%:45%)
Imunosupression
Cyclosporine (CSA)
CSA+mycophenolat-mophetil (MMF)
CONTACT:
Discussion
Results
P = NS
1. Brunstein CG et al. Marked increased risk of Epstein-Barr virus related complications with the
addition of antithymocyte globulin to a nonmyeloablative conditioning prior to unrelated
umbilical cord blood transplantation. Blood 2006; 108(8):2874-2880.
2. Parody Ret al. Severe infections after unrelated donor allogeneic hematopoietic stem cell
transplantation in adults: comparison of cord blood transplantation with peripheral blood and
bone marrow transplantation. Biol Blood Marrow Transplant. 2006;12:734-748
3. Clave E et al. Epstein-Barr virus (EBV) reactivation in allogeneic stem-cell transplantation:
relationship between viral load EBV-specific T-cell reconstitution and rituximab therapy.
Transplantation. 2004;77:76-84.
4. Wagner HJet al. Prompt versus preemptive intervention for EBV lymphoproliferative
disease. Blood. 2004;103:3979-3981.
5. Faye, A et al. Chimaeric anti-CD20 monoclonal antibody (rituximab) in post-transplant Blymphoproliferative disorder following stem cell transplantation in children. BrJ Haematol
2001; 115, 112–118.