NHL Overview
Luis E. Fayad, M.D.
Associate Professor of Medicine
Department of Lymphoma/Myeloma
U.of Texas M. D. Anderson Cancer Center
Estimated New Cancer Cases*:
10 Leading Sites, by Sex, United States, 2009
Prostate 33%
Lung & bronchus 14%
Colon & rectum 11%
Urinary bladder 6%
Melanoma of skin 4%
Non-Hodgkin’s 4%
lymphoma
Kidney 3%
Oral cavity 3%
Leukemia 3%
Pancreas 2%
All other sites 17%
32% Breast
12% Lung & bronchus
11% Colon & rectum
6% Uterine corpus
4% Ovary
4% Non-Hodgkin’s
lymphoma
3% Melanoma of skin
3% Thyroid
2% Pancreas
2% Urinary bladder
20% All other sites
*Excludes basal and squamous cell skin cancers and in situ carcinomas except urinary bladder.
Jemal et al. CA Cancer J Clin. 2003;53:5-26.
What is to Know?
Diagnosis
Staging
Prognosis
Treatment
Recurrent disease treatment
Investigational Options
Lymph Node
}
Afferent LymphaticMantle
Secondary
Marginal ZoneVessel
Zone
Germinal Center Follicle
Primary Follicle
Postcapillary
Subcapsular
Venule
Sinus
Artery
Cortex
Medullary
Cord
Medullary
Efferent
Sinus
Lymphatic Vessel
Courtesy of Thomas Grogan, MD.
Medulla
Classification of NHL
Basis of Classification
Searching for the Gold Standard
Morphology
1942
Gall &
Mallory
Morphology/
LymphomaCell Growth
Pattern
1956
Rappaport
Morphology
Immunophenotype
Cell Lineage
Genetic Features
and
Differentiation Cellular Origin Clinical Features
1970s
Kiel
1982
Working
Formulation
Date and Classification
Name
1994
REAL/WHO
Periodic Reviews
and Updates
INTO THE
FUTURE
Incidence of NHL Is Increasing,
Especially in the Elderly (60 Years)
SEER NHL incidence by age, 1975–1977 and 1998–2000 (male, all races)
140
120
1998–2000
1975–1977
100
No. per 80
100,000
60
40
20
Age at diagnosis (years)
Ries et al (eds). SEER Cancer Statistics Review, 1975-2000. National Cancer
Institute. Bethesda, Md, http://seer.cancer.gov/csr/1975_2000, 2003.
85
80–84
75–79
70–74
65–69
60–64
55–59
50–54
45–49
40–44
35–39
30–34
25–29
20–24
15–19
10–14
5–9
5
0
INTERNATIONAL WORKING
FORMULATION (1982)
Low grade
Small lymphocytic
Follicular predominantly small cleaved
Follicular mixed small cleaved and large cell
Intermediate grade
Follicular predominantly large cell
Diffuse small cleaved
Diffuse mixed small and large cell
Diffuse large cell
High grade
Large cell immunoblastic
Lymphoblastic
Small noncleaved
WORLD HEALTH ORGANIZATION
CLASSIFICATION
WHO/REAL Classification of Lymphoid Neoplasms
B-Cell Neoplasms
Precursor B-cell neoplasm
Precursor B-lymphoblastic leukemia/lymphoma
(precursor B-acute lymphoblastic leukemia)
Mature (peripheral) B-neoplasms
B-cell chronic lymphocytic leukemia / small lymphocytic
lymphoma
B-cell prolymphocytic leukemia
Lymphoplasmacytic lymphoma‡
Splenic marginal zone B-cell lymphoma
(+ villous lymphocytes)*
Hairy cell leukemia
Plasma cell myeloma/plasmacytoma
Extranodal marginal zone B-cell lymphoma of MALT type
Nodal marginal zone B-cell lymphoma
(+ monocytoid B cells)*
Follicular lymphoma
Mantle cell lymphoma
Diffuse large B-cell lymphoma
Mediastinal large B-cell lymphoma
Primary effusion lymphoma†
Burkitt’s lymphoma/Burkitt cell leukemia§
T and NK-Cell Neoplasms
Precursor T-cell neoplasm
Precursor T-lymphoblastic leukemia/lymphoma
(precursor T-acute lymphoblastic leukemia
Formerly known as lymphoplasmacytoid lymphoma or
immunocytoma
II Entities formally grouped under the heading large granular
lymphocyte
leukemia of T- and NK-cell types
* Provisional entities in the REAL classification
‡
Mature (peripheral) T neoplasms
T-cell chronic lymphocytic leukemia / small
lymphocytic lymphoma
T-cell prolymphocytic leukemia
T-cell granular lymphocytic leukemiaII
Aggressive NK leukemia
Adult T-cell lymphoma/leukemia (HTLV-1+)
Extranodal NK/T-cell lymphoma, nasal type#
Enteropathy-like T-cell lymphoma**
Hepatosplenic γδ T-cell lymphoma*
Subcutaneous panniculitis-like T-cell lymphoma*
Mycosis fungoides/Sézary syndrome
Anaplastic large cell lymphoma, T/null cell,
primary cutaneous type
Peripheral T-cell lymphoma, not otherwise characterized
Angioimmunoblastic T-cell lymphoma
Anaplastic large cell lymphoma, T/null cell,
primary systemic type
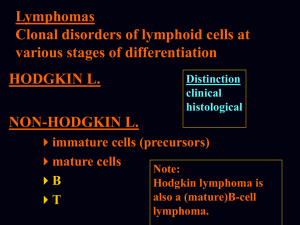
Hodgkin’s Lymphoma (Hodgkin’s Disease)
Nodular lymphocyte predominance Hodgkin’s lymphoma
Classic Hodgkin’s lymphoma
Nodular sclerosis Hodgkin’s lymphoma (grades 1 and 2)
Lymphocyte-rich classic Hodgkin’s lymphoma
Mixed cellularity Hodgkin’s lymphoma
Lymphocyte depletion Hodgkin’s lymphoma
Not described in REAL classification
Includes the so-called Burkitt-like lymphomas
** Formerly known as intestinal T-cell lymphoma
# Formerly know as angiocentric lymphoma
†
§
MATURE B-CELL NEOPLASMS
Chronic lymphocytic leukemia/small lymphocytic lymphoma
B-cell prolymphocytic leukemia
Lymphoplasmacytic lymphoma / Waldenstrom macroglobulinemia
Splenic marginal zone lymphoma
Hairy cell leukemia
Plasma cell neoplasms
Extranodal marginal zone B-cell lymphoma (MALT) lymphoma
Nodal marginal zone B-cell lymphoma
Follicular lymphoma
Mantle cell lymphoma
Diffuse large B-cell lymphoma
Mediastinal (thymic) large B-cell lymphoma
Intravascular large B-cell lymphoma
Primary effusion lymphoma
Burkitt lymphoma / leukemia
Lymphomatoid granulomatosis
MATURE T-CELL AND NK-CELL
NEOPLASMS
T-cell prolymphocytic leukemia
T-cell large granular lymphocytic leukemia
Aggressive NK-cell leukemia
Adult T-cell leukemia / lymphoma
Extranodal NK/T-cell lymphoma, nasal type
Enteropathy-type T-cell lymphoma
Hepatosplenic T-cell lymphoma
Subcutaneous panniculitis-like T-cell lymphoma
Blastic NK-cell lymphoma
Mycosis fungoides / Sezary syndrome
Primary cutaneous CD-30 positive T-cell lymphoproliferative disorders
Primary cutaneous anaplastic large cell lymphoma
Lymphomatoid papulosis
Angioimmunoblastic T-cell lymphoma
Peripheral T-cell lymphoma, unspecified
Anaplastic large cell lymphoma
IMMATURE LYMPHOID
NEOPLASMS
Precursor B-lymphoblastic leukemia/lymphoma
Precursor T- lymphoblastic leukemia/lymphoma
HODGKIN’S DISEASE (LYMPHOMA)
Nodular lymphocyte predominant
Classical
Nodular sclerosis
Mixed cellularity
Lymphocyte depleted
Lymphocyte-rich classical
PATHOLOGICAL CLASSIFICATION
General Principles
Morphology most important but does not rule
Immunophenotype is mandatory
Molecular data included when available
Clinical data included for some entities
Normal cell counterpart included for some entities
Frequency of NHL Subtypes in
Adults
Mantle cell (6%)
Peripheral T-cell (6%)
Follicular
lymphoma (22%)
Other subtypes with a
frequency 2% (9%)
Small lymphocytic
lymphoma (6%)
Composite
lymphomas
(13%)
Marginal zone
B-cell lymphoma
MALT type (5%)
Diffuse large
B-cell (31%)
Marginal zone
B-cell lymphoma
nodal type (1%)
Lymphoplasmacytic
lymphoma (1%)
Armitage JO and Weisenberger DD. J Clin Oncol. 1998;16:2780–2795.
IMMUNOPHENOTYPIC STUDIES
Flow cytometry
Quantitative
Sensitive
Immunohistochemistry
Architecture
COMMON CHROMOSOME
TRANSLOCATIONS IN NHL
Translocation
NHL
Genes Involved
t(8;14)(q24;q32)
Burkitt’s lymphoma
c-myc and IgH
t(2;8)(p12;q24)
Burkitt’s lymphoma
Igк and c-myc
t(8;22)(q24;q11)
Burkitt’s lymphoma
c-myc and Igλ
t(11;14)(q13;q32)
Mantle cell lymphoma
cyclin D1 and IgH
t(14;18)(q32;q21)
Follicular lymphoma
IgH and bcl-2
t(3;14)(q27;q32)
Diffuse large B-cell lymphoma
bcl-6 and IgH
t(11;18)(q21;q21)
Low-grade B-cell MALT lymphoma api-2 and mlt
t(1;14)(p22;q32)
Low-grade B-cell MALT lymphoma bcl-10 and IgH
t(2;5)(p23;q35)
T-anaplastic large cell lymphoma
alk and npm
t(9;14)(p13;q32)
Nodal B-cell lymphoma
pax-5 and IgH
RELATIVE FREQUENCY OF NHL
Common
Diffuse large B-cell
Follicular
Small lymphocytic
Mantle cell
Peripheral T-cell, NOS
Marginal zone B-cell, MALT
31 %
22 %
6%
6%
6%
5%
Nebraska NHL Classification Project
J Clin Oncol 16: 2780, 1998
REACTIVE FREQUENCY OF NHL
Uncommon
Primary mediastinal large B-cell
Anaplastic large cell, T/null
Lymphoblastic (mostly T)
Burkitt-like
Marginal zone B-cell, nodal
Lymphoplasmacytic
Burkitt and other types
2%
2%
2%
2%
1%
1%
<1%
J Clin Oncol 16: 2780, 1998
Staging
Physical examination
Blood testing
–
–
–
–
Cell count and differential
Comprehensive chemistry pannel (LDH), immunoglobulin, SPEP,
immunofixation, light chains
Beta-2 microglobulin
HIV, hepatitis profile, HTLV-1
CT scans
PET scans Standard in aggressive lymphomas, Hodgkin’s (indicated in
some indolent as well)
Cardiac evaluation
Peripheral blood flow-cytometry
Molecular studies, Coombs test, and others
Bone marrow aspiration and biopsies
Occasional Pulmonary tests, etc
CNS lymphoma, MRIs and ophtalmology
If clinically indicated lymbar puncture and skin biopsies
Response
Physical
Blood work
Scans
Bone marrow biopsy
MRIs, lumbar puncture, as indicated
PET scan often indicated (ELABORATE
FURTHER)
Most Frequent
DLBCL
Follicular Lymphomas (remark in
transformation)
Discordant histologies
Mantle cell lymphoma
Small lymphocytic lymphomas
PTCL
Today’s Management of DLBC
Lymphomas
Diagnosis
Prognostic factors
Current treatments
Salvage Regimens
Future Strategies
Adverse Features in the
International Prognostic Factors Index
A
P
E
L
S
AGE >60
PERFORMANCE STATUS
EXTRANODAL >1
LDH >NL
STAGE III-IV
International Prognostic Index (IPI)
Stratifies Risk by Clinical Factors in Aggressive NHL
Prognostic factors (APLES)
• Age 60 years
• Performance status 1
• LDH 1 normal
• Stage III or IV
• Low
Factors
0 or 1
• Low-intermediate
2
• High-intermediate
3
• High
Patients (%)
• Extranodal sites 1
Risk category
Overall survival
100
L
LI
HI
H
50
0
0
2
4 or 5
International non-Hodgkin’s lymphoma prognostic factors project. N Engl J Med. 1993;329:987.
4
6
8
10
International Prognostic
Index:
Age Adjusted
Prognostic factors
• Performance status 1
• LDH 1 normal
Factors
• Low
0
• Low-intermediate
1
• High-intermediate
2
• High
3
Patients (%)
• Stage III or IV
Risk category
Overall survival
100
L
LI
HI
H
50
0
0
2
International non-Hodgkin’s lymphoma prognostic factors project. N Engl J Med. 1993;329:987.
4
6
8
10
R-IPI: Risk groups remain in DLBCL
in the rituximab era
28
Retrospective analysis of an
unselected population of pts
with DLBCL (N= 365)
All DLBCL pts received R-CHOP:
45% were high-risk (IPI 3-5)
50% of high-risk pts relapsed
within 4 yrs
IPI score remains an
important prognostic
and predictive tool in
the rituximab era
Sehn et al., Blood. 2007;109:1857
Double Hit Lymphomas
IgH-BCL-2 and c-myc rearrangements
Novel category in WHO 2008
BCL2/C-MYC
BCL6/C-MYC
BCL2/BCL6/C-MYC (Triple Hit)
Cyclind D1/C-MYC
BCL3/MYC
9p13/MYC
9p13/MYC/BCL3 Triple
C-MYC
Findings are ?? On overall lymphoma
population
Different series, selection bias to do the
test
Outcomes depending of associated
genetic abnormalities
Incidence of 3%-16%
Localized Lymphoma – SWOG 8736
Overall Survival by Stage-Modified IPI
100
Percent
80
60
40
P <0.001
5-Yr
N Death estimate
14
121
94%
0 Risk factor
94
73%
1 Or 2 risk factors 250
23
30
50%
>2 Risk factors
20
0
0
3
6
9
Years after randomization
Miller TP, et al: Blood 98:724a, 2001
12
R-CHOP for the elderly
(GELA)EFS and OS– Median followup 7 y
Overall survival according to
aaIPI
Low risk patients
High risk patients
MInT Trial: TTF
100
% of patients
Rituxan® (Rituximab) + chemo
n=413
80
60
n=410
Chemo
40
Median observation time: 22 months
P<0.0001
20
0
HR=0.45 (95% CI, 0.34-0.59)
0
5
10
15
20
25
Months
Data on file, Genentech.
30
35
40
45
50
MInT Trial: Overall Survival
100
Rituxan® (Rituximab) + chemo
% of patients
n=413
80
Chemo
n=410
60
Median observation time: 23 months
in surviving patients
40
P=0.0002
HR=0.40 (95% CI, 0.24-0.66)
20
0
0
5
10
15
20
25
Months
Data on file, Genentech.
30
35
40
45
50
Risk of Relapse
Front-line therapy dictates NHL treatment success.
Chart taken from Coiffier B. Treatment of Aggressive Lymphomas Disseminated Cases 2003 ASCO Mtg.
19
FDG-PET scan Predicts
Response to SCT
100%
86.70%
83.30%
80%
60%
40%
20%
0%
13.30%
CR REL
10%
0%
PET+ (n=30)
Spaepen K et al. Blood. 2003; 102:53-59
CR REL
6.70%
DonCR
PET- (n=30)
CORAL: COllaborative trial in Relapsed
Aggressive Lymphoma
R-ICE versus R-DHAP in relapsed patients
with CD20 diffuse large B-cell lymphoma
(DLBCL) followed by autologous stem cell
transplantation: CORAL study.
C. Gisselbrecht, B. Glass, N. Mounier, D. Gill, D. C. Linch, M. Trneny,
A. Bosly, O. Shpilberg, H. Hagberg, N. Ketterer, D. Ma, P. Gaulard,
C. Moskowitz, and N. Schmitz.
CORAL Trial of RICE v DHAP
Which salvage regimen is the best?
R
A
N
→
D
CD20+ DLBCL
O
Relapsed/Refractory
M
I
Z
E
SD/POD → Off
R-ICE x 3
AB
SE
CA
TM
R-DHAP x 3
R
A
Rx6
N
D
PR/CR →
O
M
I
Z
Obs
E
N=400
Place of immunotherapy post transplantation?
Orlando ASCO May 2009 / Coral study C. Gisselbrecht
56%
56%
45%
OVERALL SURVIVAL
ACCORDING TO TREATMENT
ARM (INDUCTION ITT)
PROGRESSION-FREE
SURVIVAL ACCORDING TO
TREATMENT ARM
(INDUCTION ITT)
42%
Orlando ASCO May 2009 / Coral study C. Gisselbrecht
DLBCL Recurrent after SCT or no
Canditates
Consider investigational trials
Allogeneic transplantation in selected
cases (ELABORATE)
Palleative treatment
COnfort care
Follicular Lymphomas
Often advanced at diagnosis
Mutation BCL-2 in 80%
Transformation
Watchful waiting
Recurrent disease
Allogeneic transplant or autologous
FOLLICULAR LYMPHOMA
Grading
Grade
I
(small cleaved)
No. centroblasts / hpf
<5
II (mixed)
5-15
III (large cell)
>15
Follicular Lymphoma Grade 1
2
1a
Small cleaved lymphocytes in
nodules
Courtesy of Randy D. Gascoyne, MD.
Follicular Lymphoma Grade 2
1
2
Small cleaved cells and large uncleaved cells in
nodules
Courtesy of Randy D. Gascoyne, MD.
Risk of Histologic
Transformation in NHL
100
Probability (%)
Treated Upon Diagnosis (n=131)
80
Initially Untreated
(n=83)
60
40
20
0
0
2
4
6
Horning et al. N Engl J Med. 1984;311:1471-1475.
8
10
12
14
16
18
20
Improvement in Survival of Stage IV Indolent NHL at
MD Anderson 1977-2003
100
--FND-R+IFN vs FND->R +IFN
--ATT->IFN vs FND->IFN
--ATT->IFN
--CHOP-Bleo+IFN
--CHOP-Bleo
75
%
P=0.005
P=0.04
P=0.19
P=0.05
50
25
0
5
10
20
15
25
30
Years
Liu Q et al, Blood 2003; 102: 398a
The Challenge of Follicular NHL
Variable presentation and prognosis
Long survival: In general, there is no cure
Rituximab is a major advance: But not “curative”
Multiple therapies: No standard, how best to sequence
Many novel therapies in development
Reliable biomarkers needed
Follicular lymphoma
Accelerated
Indolent
Transformation
10%–15%
40%–65%
20%–60%
Images courtesy of Stephanie Gregory, MD.
NHL = non-Hodgkin’s lymphoma.
Tan et al, 2008; Berglund et al, 2007; Skarin et al, 1997.
Chemotherapy Rituximab Trials for
Initial Therapy of Follicular NHL
Hiddemann
Czuczman
100
90
80
70
60
50
40
30
20
10
0
Zinzani 2
Zinzani 1
Marcus
McLaughlin
Herold
Czuczman
PR
CR
CHOP-R CHOP-R
CHOP-R
FN-R
FND-R
CVP-R
F-R
MCP-R
PR = partial response; FN-R = fludarabine, mitoxantrone, rituximab; FND-R = fludarabine, mitoxantrone, dexamethasone, rituximab;
F-R = fludarabine, rituximab; MCP-R = mitoxantrone, chlorambucil, prednisone, rituximab.
Czuczman et al, 1999; Hiddemann et al, 2005; Zinzani et al, 2004; Marcus et al, 2005; McLaughlin et al, 2000; Czuczman et al, 2005;
Herold et al, 2007.
Follicular Lymphoma Challenges
Maintenance
Maintenance dosing
Transplant when and who
Too many options
Transformation
New drugs
Small Lymphocytic Lymphoma
1
2
3
4
Small round lymphocytes; proliferation centers
Courtesy of Randy D. Gascoyne, MD.
MALT-Type Lymphoma (Extranodal MZL)
1
2
3
4
Courtesy of Randy D. Gascoyne, MD.
SLL/CLL
A
Courtesy of Randy Gascoyne, MD.
B
Probability of Disease Progression
100
Patients Treated (%)
80
60
40
17p deletion
11q deletion
12q trisomy
Normal
13q deletion as
sole abnormality
20
0
0
12 24 36 48 60 72 84 96 108 120 132 144 156 168 180
Months
Dohner et al. N Engl J Med. 2000;343:1910-1916.
Probability of Survival
100
17p deletion
11q deletion
12q trisomy
Normal
13q deletion as
sole abnormality
Patients Surviving (%)
80
60
40
20
0
0
12 24 36 48 60 72 84 96 108 120 132 144 156 168 180
Months
Dohner et al. N Engl J Med. 2000;343:1910-1916.
Un-mutated Ig VH Genes:
More Aggressive CLL (N=84)
Stage
A: 62 B: 9
C:
PD:SD
34:50
Unmutated
38 (45%)
Mutated
46 (55%)
Hamblin et al. Blood. 1999;94:1848-1854.
13
The Promoting Roles of Antigen Stimulation and Accessory Signals from the
Microenvironment in CLL
Chiorazzi, N. et al. N Engl J Med 2005;352:804-815
Comparison of CLL Patients With
Mutated and Unmutated VH Genes
100
90
Mutated
% Surviving
80
Unmutated
70
60
50
40
30
20
10
0
0
25
50
75
100
125 150
175 200
Months
Hamblin et al. Blood. 1999;94:1848-1854.
225 250
275 300 325
Comparison of Stage A CLL Patients
With Mutated and Unmutated VH Genes
100
90
Mutated
% Surviving
80
Unmutated
70
60
50
40
30
20
10
0
0
25
50
75
100
125 150
175 200
Months
Hamblin et al. Blood. 1999;94:1848-1854.
225 250
275 300 325
FCR Survival by Rai Stage
1.0
Proportion Alive
0.8
0.6
0.4
Pts. Died Rai Stage
106 23 0, I
93 23
II
44 16
III
57 17
IV
0.2
0.0
0
1
2
3
Keating, M., Personal Communication
4
5
Years
6
7
8
9
Other NHL
Mantle cell lymphoma: Front line
transplant, HCVAD, new drugs, velcade
MALT stomach, antibiotics? Radiation?
CNS lymphoma very complicated
T-cell lymphomas ALCL ALK-positive
PTCL Other
CTCL
THANKS