The role of cholinergic balance in immune responses: a novel anti
advertisement

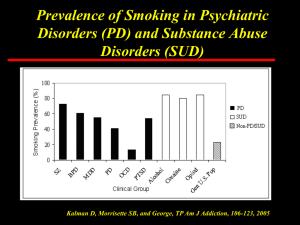
Cholinergic anti-inflammatory signaling through α7 nAChR Talma Brenner Department of Neurology Hadassah University Hospital Jerusalem, Israel 1 Α 7 nicotinic acetylcholine receptor • The α 7nAChR an anti-inflammatory target in macrophages and T-cells (B-cells) • α 7nAChR presence on immune cells may explain epidemiological data claiming link between cigarette smoking and several inflammatory disorders (ulcerative colitis, sarcoidosis) • Animal studies show that nicotine is active in reducing Ab production and in signaling through the TCR • Involvement of the immune cholinergic system in the regulation of autoimmune responses 2 Non-neuronal immune cholinergic system • Synthesis of ACh • Expression of the enzyme cholineacyltransferase (ChAT) • Response to cholinergic signals; muscarnic and nicotinic • Expression of α7 nicotinic acetylcholine receptor (AChR) • Termination of cholinergic signals is mediated by AChE 3 nAChR – structure and function • Neuronal nAChR are pentameric extracellular complexes of α and β subunits that form a ligand gated ion channel. (9 α and 4 β subunits). 4 Neuro-immune interactions * The nervous system is a major producer of ACh, the immune cholinergic system can mediate neuro-immune interactions * or serve as an internal regulator of immune responses * α7nAChR on macrophages and Tcells can be antiinflammatory target 5 Neuroimmune interactions (The cholinergic anti-inflammatory surveillance) 6 (Ulloa, 2007) AChE: Termination of cholinergic signaling A12 G4 G2 7 G1 G2 G1 AChE Inhibitors (AChEI) * Clinical indications: Alzheimer’s Disease (AD) and Myasthenia Gravis (MG). * Inhibitors of AChEI enzymatic activity (Conventional): Donepezil, Rivastigmine, Tacrine, Pyridostigmine and Edrophonium. * Inhibitors of AChE synthesis: EN101, antisense targeted to exon 2 of the AChE mRNA. 8 Anti-sense directed to ACHE mRNA (EN101) treatment in experimental MG 9 Brenner et al FASEB J. Oral EN101 improves decremental response in EAMG rats 10 Brenner et al FASEB J. Chronic EN101 Retards EAMG Progression Stamina Disease Severity . . . (sec ) Running time Clinical score . . Be fore Saline Me stinon EN Before Mestinon EN - (g ) Weight change Body Weight (% ) -day survival Survival Saline Saline 11 Mestinon EN Saline Mestinon EN Suppression of Anti-AChR abs and muscle chemokine/chemokine receptor by chronic EN 101 in EAMG rats CXCR3 expression (muscle) Anti-rat-AChR abs * 20 16 * AU ** 12 8 80 4 Pmole /ml 0 60 40 Control EAMG EAMG+EN101 ** IP10 expression (muscle) 20 * 16 0 Saline Control EAMG EAMG +EN101 12 8 4 0 12 Control EAMG EAMG+EN101 EN 101 in phase Ib in MG Mean change in QMG 0 Mean Change -1 -2 Baseline -3 -4 -5 -6 -7 3.5h after first dose 3.5h after 2nd dose 3.5h after 3rd dose Day 2 Day 3 Day Day 4 Day 5 Day 6 On EN101 500 µg/kg, day 2 4 wk after treatment 13 Argov et al Our working hypothesis: Activation of the 7 nAChR has anti-inflammatory effects 7 nAChR ACh 7 nAChR ACh AChEI AChE AChE AChE AChE AChE AChE Cholinergic up-regulation by inhibition of (extra-cellular) AChE can activate the 7 nAChR 14 Reduction in AChE activity in the extracellular medium of proliferating T-cells by AChEI Relative AchE Activity (O.D. 405nm) 1.0 PHA PHA+EN101 1µM 0.9 PHA+EN101 2µM 0.8 PHA+edrophonium 0.5µM PHA+edrophonium 1µM 0.7 0.6 0.5 0.4 0.3 0.2 0.1 0.0 5 10 15 25 Minutes in Substrate 15 30 7 nAChR is specifically up-regulated during T-cell activation 7 nAChR involvement in T-cell proliferation cholinergic up-regulation inhibits T-cell proliferation and can render activated cells to be more sensitive to nicotinic agonists 16 Nizri et al Neuropharmacology 2006 Attenuation of pro-inflammatory cytokine production by 7 nAChR activation 17 Nizri et al Neuropharmacology 2006 Expression of α7 nAChR by CD4+ T-cells: increase by stimulation and inhibition by α7 AS 18 Isolated CD4+ cells stained with FITC αbgtx Nizri et al J. Immunol 2009 Cells positive for α-Bgtx are activated cells in replication 19 Up-regulation of α7nAChR in EAMG mice by Torpedo-AChR 4 3.5 fold of change 3 2.5 2 1.5 1 0.5 0 ctl 20 torpedo What is so special about 7 nAChR? CD3 and 7 nAChRs coimmunoprecipitate (Razani-Boroujerdi , 2007) 21 22 Attenuation of EAE by AChEI (rivastigmine) Subcutaneous daily injection Delivery by mini osmotic pumps Treatment control rivastigmine 2.2 ± 0.4 0.6 ± 0.1** 48.8 ± 5.1 13.5 ± 5.8*** Treatment control rivastigmine rivastigmine+ Mecamyl. Mean severity 3 .4± 0.4 1.7 ± 0.3 * 2.9 ± 0.1 Mean severity Cumulative score# 70.4 ± 4.8 32.4 ± 2.7 * 50.8 ± 4.2 * Cumulative score # Inhibition of AChE activity: Brain 56%, Muscle 42% 23 Nizri et al .J Neuroimmunology, 2008 Histological preservation of the CNS following AChEI treatment 24 Nizri et al J. Neuroimmunology, 2008 Quantification of histopathological parameters Pathological parameter Inflammation Demyelination Axonal damage 25 control (n=6) rivastigmine (n=7) 2.3 ± 0.49 1.8 ± 0.47 1.8 ± 0.37 0.71 ± 0.45* 0.71 ± 0.24* 0.57 ± 0.35* The inflammatory demyelinating lesions in EAE and MS 26 Reduction of Th1 and Th17 activity by AChEI treatment in MOG-specific T-cells 27 AChEI treatment affect antigen presentation 28 Direct signaling of α7 nAChR by nicotine 29 Signaling downstream of 7 nAChR 30 Inhibition of Signaling downstream of 7 nAChR by nicotine 31 Nizri et al 2009 Direct activation of 7 nAChR by nicotine control p cumulative score 70±8.2 17±7.1* 0.0011 mean severity 3.3±0.4 0.8±0.1* 0.0001 16 15.7 0.02 onset 32 nicotine Nizri et al J. Immunology 2009 Improved histophatological parameters under nicotine Demyelination PBS Nicotine 33 treatment Microglial activation Axonal damage Reduced CD4 and CD11b cells in the CNS of EAE mice treated with nicotine 34 Nicotine treatment was associated with reduced T-cell reactivity 35 Α7 KO mice develop milder EAE without response to nicotine treatment Disease Mean severity WT 3.3±0.4 Α7-/1.5±0.2 Α7 -/- w nicotine 1.4±0.1 36 Altered T-cell reactivity in α7 nAChR KO, lack of response to nicotine and reduction in antigen presentation 37 Does nicotine posses antiinflammatory effects? • Invasive pneumococcal disease is more prevalent in smokers (Nuorti, 2000). Nicotine increases intracellular replication of Legionella (Matsunaga, 2001). • Smoking AD patients have reduced levels of Aβ 40 and Aβ 42 (Hellstrom-Lindahl, 2004). • Smoking protects against UC (Rubin and Hanauer, 2000). Clinical trials with nicotine in TDP reduced UC severity (Van Assche, 2005). 38 History may be revealing… 39 Inhibition of AChE by Rivastigmine reversed cognitive impairment associated with EAE Assay period induction 0 40 clinical signs 7 14 EAE is associated with inflammatory infiltrates near the hippocampus 41 Cognitive dysfunction in MS • Most MS patients develop cognitive dysfunction (memory, attention, information processing and verbal fluency). • These patients are more prone to develop affective disorders (Gilchrist and Creed, 1994). • Immunmodulatory treatment can slow the cognitive decline in MS, there’s still a need for symptomatic treatment (Henze, 2006). • AChEI are prescribed for cognitive dysfunction in AD. Recent evidence pointed to their efficacy in MS patients (Krupp 2004). 42 43 Conclusions I The immune cholinergic system can be harnessed for immunomodulation Proposed mechanism involves activation of α7 nAChR Cholinergic up-regulation by AChEI treatment results in reduction of neuroinflammation, amelioration of EAE and improvement of cognitive deficit AChEI mediate anti-inflammatory effects; Suppression of T-cell activities, proliferation, pro-inflammatory cytokine production 44 Conclusions II α7 nAChR is expressed on CD4+ cell surface. Expression increases following stimulation Nicotine treatment suppressed EAE Nicotine treatment reduced T-cell reactivity, Th1 and Th17 activity and implicated a skew towards Th2 with inhibition of NFkb induced signaling Effects of nicotine are α7 nAChR dependent Differential responses of α7 nAChR-/-cells; impairment in antigen presentation and increased T-cell proliferation 45 Thanks • • • • • • • Lab members Eran Nizri Michal Irony-TurSinai Yasmine HamraAmitay Neli Boneva Camille Sicsic Omer Lori Hodaya Hadad 46 • • • • • • • • Collaborators M. Rosin E. Lavi S. Berrih-Aknin H. Soreq O. Abramsky Ester\ Neuroscience AFM Israeli Ministry of Health chief scientist fund