B2-Fri-2-5-Herst
advertisement

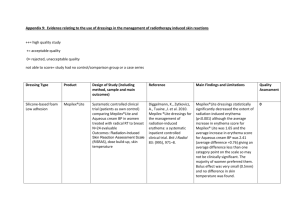
Fri 30th Aug 2013 Session 2 / Talk 5 11:40 – 12:00 BROOKLYN 2 RADIOTHERAPY Patries HERST ABSTRACT Purpose: Severe acute radiation-induced skin reactions occur in a significant proportion of women who receive radiation therapy for breast cancer. We previously showed that soft silicone dressings decreased the severity of severe skin reactions by 40% when applied from the onset of erythema. Here we report the effect of a transparent silicone film on the severity of skin reactions and the incidence of moist desquamation when used from the beginning of treatment in breast cancer patients. Methods and Material: A total of 80 women were recruited from Dunedin Hospital. The breast or chest wall was randomly divided into lateral and medial halves at the start of radiation therapy treatment; one half was treated with Mepitel Film, the other half with aqueous cream. Skin reactions were assessed using the Radiation-Induced Skin Reaction Assessment Scale. Results: Mepitel Film prevented the onset of moist desquamation in all patients and decreased the severity of skin reactions by more than 80%. Patients preferred the film and found it very comfortable to wear. Conclusions: Mepitel Film prevents moist desquamation when used prophylactically Mepitel Film for Radiation-Induced Skin Reactions Dean Paterson, Prashika Poonam, Noelle Bennett, Annie Sutherland, Ruth Peszynski, Meredith van Beekhuizen, Katie Diggelmann, Marieke Jasperse, Patries Herst NZIMRT, 2013 Rationale Unavoidable side effect of external beam RT No standard treatment Form protective barrier Inert Adhere to healthy skin but do not stick to open wounds Decrease the severity of erythema by 30% in a pilot study of 30 breast cancer patients. (Diggelmann et al. Br J Radiol 83, 2010) Stage III RCT: Mepilex Lite: Management Trial Auckland (ARO), Palmerston North, Wellington, Dunedin 80 patients enrolled, 74 analysed Erythema Eligibility • Post-mastectomy • No metastatic disease • No previous RT to chest In-Patient Randomization • Able to attend follow up • Good performance status Mepilex Aqueous cream 1] severity Assessment 2] % MD 3] time to MD RISRAS • 3x a week during TMT • 1x a week for 4 weeks after TMT completion Mepilex Moist desquamation Standard dressings 4] time to healing Healing RISRAS (total scores between 0 and 36)* Researcher Component (total scores between 0 and 24) Erythema (E) 0 Normal skin 1 Dusky pink 2 Dull red 3 Brilliant red 4 Deep redpurple Dry Desquamation (DD) 0 Normal skin 1 (<25%)** 2 (25%-50%) 3 (50%-75%) 4 (>75%) Moist Desquamation (MD) 0 Normal skin 1.5 (<25%) 3.0 (25%-50%) 4.5 (50%-75%) 6 (>75%) Necrosis (N) 0 Normal skin 2.5 (<25%) 5.0 (25%-50%) 7.5 (50%-75%) 10 (>75%) Patient Component (total scores between 0 and 12) Symptoms Not at all A little Quite a bit Very much Do you have any tenderness, discomfort of pain of your skin in the treatment area? 0 1 2 3 Does your skin in the treatment area itch? 0 1 2 3 Do you have a burning sensation of your skin in the treatment area? 0 1 2 3 To what extent has your skin reactions and your symptoms affected your day to day activities? 0 1 2 3 RISRAS Results of 74 mastectomy patients Decrease in overall skin reaction severity: 40% p<0.001 Paterson et al., Journal of Cancer Science and Therapy 4(11) 347-356 (2012) Moist Desquamation Results Decreased the area of MD by 49% Did not affect % MD or time to MD/healing Fall off in shower or when perspiring, do not stick well in axilla Are not transparent: can’t see tattoos Have a small bolus effect (0.5mm), so must be removed during RT We need something better: Mepitel Film? Same features as Mepilex Lite Much thinner, transparent and more sticky than dressings and negligible bolus effect (0.1mm) Lateral Medial Stage III RCT: Mepitel Film Dunedin: 80 patients (both breast and mastectomy) Eligibility Start of RT treatment • No metastatic disease • No previous RT to chest In-Patient Randomization • Able to attend follow up • Good performance status Mepitel Film Aqueous cream Assessment RISRAS • 3x a week during TMT • 1x a week for 4 weeks after TMT completion 1] severity 2] % MD Moist desquamation RISRAS Scores of 78 patients Decrease in severity of skin reactions: 92% P<0.001 Moist Desquamation Results Treatment # Patches Mastectomy MD % MD Aqueous Cream (n=34) Mepitel (n=34) 8 0 24% 0% No mastectomy Aqueous Cream (n=44) Mepitel (n=44) 12 0 27% 0% Total Aqueous Cream (n=78) Mepitel (n=78) 20 0 26% 0% No Moist Desquamation under the Film No Moist Desquamation under the Film Interesting finding Stressed patients have worse skin reactions Conclusions Mepilex Lite Mepitel Film Trial Type Management Prevention RISRAS < 40% < 90% % MD Same as control No MD at all Patients “Very comforting and easy to use” “Film? What film? I forgot it was there” Is Mepitel Film the answer to RT-induced acute skin reactions? Try it out and let me know…… Acknowledgements Trial Participants Staff from Departments: Southern DHB Capital and Coast DHB Mid-Central DHB Auckland Regional Oncology Funding Rouse Educational Trust Difference not due to differences in dose between Film and cream covered skin. Areas measured and compared: Axilla (2TLDs) IFM fold (4 TLDs) Superior-medial (5 TLDs) Inferior Lateral (5 TLDs)