Appendix 9 - Dressings table
advertisement
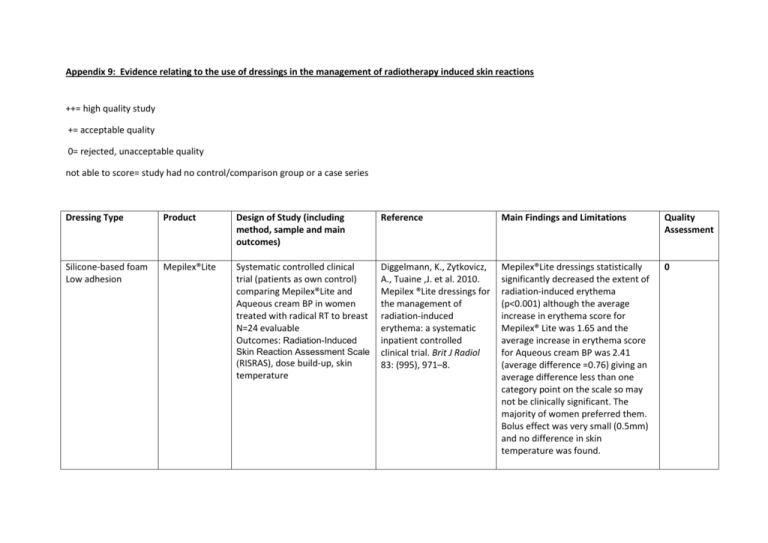
Appendix 9: Evidence relating to the use of dressings in the management of radiotherapy induced skin reactions ++= high quality study += acceptable quality 0= rejected, unacceptable quality not able to score= study had no control/comparison group or a case series Dressing Type Product Design of Study (including method, sample and main outcomes) Reference Main Findings and Limitations Quality Assessment Silicone-based foam Low adhesion Mepilex®Lite Systematic controlled clinical trial (patients as own control) comparing Mepilex®Lite and Aqueous cream BP in women treated with radical RT to breast N=24 evaluable Outcomes: Radiation-Induced Diggelmann, K., Zytkovicz, A., Tuaine ,J. et al. 2010. Mepilex ®Lite dressings for the management of radiation-induced erythema: a systematic inpatient controlled clinical trial. Brit J Radiol 83: (995), 971–8. Mepilex®Lite dressings statistically significantly decreased the extent of radiation-induced erythema (p<0.001) although the average increase in erythema score for Mepilex® Lite was 1.65 and the average increase in erythema score for Aqueous cream BP was 2.41 (average difference =0.76) giving an average difference less than one category point on the scale so may not be clinically significant. The majority of women preferred them. Bolus effect was very small (0.5mm) and no difference in skin temperature was found. 0 Skin Reaction Assessment Scale (RISRAS), dose build-up, skin temperature Silicone- based foam Low adhesion Silicone -based foam Low adhesion Mepilex®Lite Mepilex®Lite Randomised controlled trial (patients as own control) comparing Mepilex®Lite and Aqueous cream BP on first area of erythema to appear during radical RT to breast post mastectomy. N=74 evaluable patients. Outcomes: RISRAS, time to moist desquamation, time to healing Paterson, D., Poonam, P., Bennett, N., et al. 2012) Randomized intra-patient controlled trial of Mepilex ®Lite dressings versus Aqueous cream BP in managing radiationinduced skin reactions postmastectomy. JCancer Sci Ther 04:347-56. Randomised controlled trial comparing the effectiveness of Mepilex® Lite dressings and usual care (described as wound care and cleansing with normal saline) in patients undergoing chemo-radiation for nasopharyngeal carcinoma. NB preliminary results also reported in Bennett N et al. 2013. Proceedings from RTi3 2013: Inquire, Inspire, Innovate/Journal of Medical Imaging and Radiation Sciences 44 (2013) 44-58 Zhong, WH., Tang, QF., Hu, LY., Feng, HX. 2013. Mepilex® Lite dressings for managing acute radiation dermatitis in nasopharyngeal carcinoma patients: a systematic controlled clinical trial. Limitations: small trial, RISRAS measurements stopped when dry desquamation occurred, so effect on more severe skin reactions not assessed. No blinding of assessors and no allocation concealment. Mepilex®Lite dressings did not significantly reduce the incidence of moist desquamation, but reduced the overall severity of skin reactions by 41% (p < 0.001) and the average moist desquamation score by 49% (p = 0.043). 80% of participants said they preferred the dressings over the cream and RISRAS scores for pain/discomfort, itchiness, burning and effect on day to day life were significantly better in the Mepilex®Lite area. Limitations: study only compared dressings applied to the first area of erythema to appear, which was not always the worst affected area overall. Radiation-induced dermatitis in the Mepilex® Lite group healed significantly more quickly than in the control group (median 16 days vs. 23 days, p=0.009). Although on multivariate analysis, dressing type was no longer a significant determinant of time to wound + 0 Hydrophilic Polyurethane Foam Non-adhesive Silicone-based transparent film PolyMem® Mepitel® film N = 88 Outcome: Time to wound healing; RISRAS, wound pain, restriction of neck movement, sleep and body image disturbance. Medical Oncology 30: (4), 761. Case studies of PolyMem® in patients with erythematous radiation skin reactions (RTOG >1) undergoing RT for head and neck cancer N = 20 Outcomes: pain and sleep diary, healing Scott, A. 2014. Polymeric membrane dressings for radiotherapy-induced skin damage Br J Nurs(Oncology Supplement), 23(10), S24, 26-31 Randomised controlled trial comparing prophylactic Mepitel® film with Aqueous cream BP (patients as own control) during RT for breast cancer N = 80 Outcomes: skin reaction severity, incidence of moist desquamation healing. Sleep, pain and combined average researcher and patient RISRAS scores were significantly better in the Mepilex®Lite group. Limitations: as with other studies, this was not blinded. It was not entirely clear at what point the dressings were first applied. Hers, P., Bennett, N., Sutherland, A., Peszynski, R., Paterson, D., Jasperse, M. 2014. Prophylactic use of Mepitel® film prevents radiation-induced moist desquamation in an intrapatient randomised controlled clinical trial of 78 breast cancer patients. Radiotherapy and Oncology 110:1, 137-143. Pain and sleep scores improved Not able to during the radiotherapy treatment score course after PolyMem® dressings had been applied. Skin had healed within a week in 8 patients, and within 3 weeks in another 7 patients. Patients and carers were able to change the dressings themselves. Limitations: small, uncontrolled study The study reported 0% moist 0 desquamation rates for the Mepitel® covered areas and 26% for the control areas (p<0.0001). Mepitel® film significantly decreased the combined, researcher and patients RISRAS scores (p < 0.0001) by 92% (compared with the Aqueous cream BP areas). The majority of patients (n=55) preferred Mepitel® film to cream because it felt comfortable and protective. Limitations: as with other ‘within patient’ trials, it was impossible to blind the trial. Non adhesive Silver clear nylon dressing (SCND) Randomised controlled trial – Phase III study comparing silver clear nylon dressing with sulfadiazine cream in patients with rectal or anal cancer receiving external beam RT at the point grade 1dermatitis became present. N = 40 Outcome: skin toxicity on final day of treatment Niazi, T., Vuong, T., Azoulay, L., Marijnen, C., Bujko, K., Nasr, E. et al. 2012. Silver clear nylon dressing is effective in preventing radiationinduced dermatitis in patients with lower gastrointestinal cancer: results from a phase III study. Int J Radiat Oncol Biol Phys 1: 84:e305–e310. Mean dermatitis score on the last day of RT (assumed to be the point at which skin reactions were at their worst) was 2.53 (standard deviation [SD], 1.17) in the standard arm and 1.67 (SD, 1.2; P=.01) in the SCND arm. 2 weeks after RT, the difference was 0.39 points in favour of SCND (non-significant). Limitations: This was a small single blind study. There were differences between the histological diagnosis of the patients and the concurrent chemo/ radiotherapy regimes. No patient reported outcomes were used. ++







