M. tuberculosis
advertisement

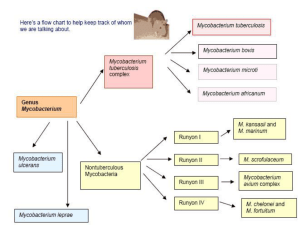
Microbiology 2011 5/11/2011 Yu Chun-Keung Chapter 28 Mycobacterium Chapter 27 Nocardia Actinomyces Tuberculosis Tubercular decay has been found in the spines of Egyptian mummies. Pictured: Egyptian mummy in the British Museum [Source: Wikipedia] One new case / 36 min One death / 6 hours Genus Mycobacterium (分枝桿菌屬) Nonmotile, non-spore-forming aerobic bacilli Ubiquitous presence, 130 species; 7 species cause most infections Pathogenic Mycobacteria M. tuberculosis (結核分枝桿菌) : airborne M. leprae (痲瘋分枝桿菌) : close contact M. avium complex : water/soil M. kansasii : water/soil M. fortuitum : water/soil M. chelonae : water/soil M. abscessus : water/soil Cell Wall of Mycobacteria G(+) bacteria Inner cytoplasmic membrane Anchor : proteins, phosphotidylinositol mannosides, lipoarabinomannan (LAM) Thick peptidoglycan layer Attach arabinogalactan (a branched polysaccharide) + mycolic acid (70-90 carbon) No outer membrane Cell Wall of Mycobacteria Rich in lipids: responsible for many characteristic properties (acid-fastness, resistant to disinfectants and antibiotics, antigenicity, slow growth, clumping), 60% of dry weight. Acid-fastness: mycolic acid -resistant to common laboratory stain. Once stained, cannot be decolorized with acid solutions Polypeptides: transport proteins and porins; 15% dry weight; PPD (purified protein derivatives) - induce cell-mediated immunity. Preliminary classification of mycobacteria by growth properties and colonial morphology 1. M. tuberculosis complex slow-growing, no pigmentation 2. Non-tuberculous mycobacteria (NTM) Runyon group I slow-growing, yellow pigment (+) in light Runyon group II slow-growing, yellow pigment (+) in dark Runyon group III slow-growing, nonpigmented Runyon group IV rapidly growing A pigmented and / or a rapidly growing mycobacterium M. tuberculosis 1. Mycobacterium tuberculosis complex Tuberculosis M. tuberculosis (airborne/droplet) M. bovis (unpasturized products) milk M. tuberculosis - pathogenesis Inhalation of aerosolized infectious particles to alveoli Intracellular pathogen, infect inactivated alveolar macrophages, lifelong infections Prevent phagosome-lysosome fusion by blocking early endosomal autoantigen 1 (EEA1) Acquire nutrients from other intracellular vesicle Resist killing by nitric oxide and superoxide anions Induce protective immunity: macrophages release IL-12 and TNFα, and recruit T cells and NK cells; induce TH1 cell; secrete IFN-γ; activate macrophages; increase intracellular killing Infected cells spread locally (lymph nodes) or hematogenous spread (bone marrow, spleen, kidneys, CNS), and attract macrophages and lymphocytes; formation of granuloma Caseous necrosis (乾酪壞死) Granuloma (肉牙腫) Inner mass : alveolar macrophages, epithelioid cells, Langhans giant cells (fused epithelioid cells) Dense wall : CD4, CD8, NK, T cells, macrophages Caseous necrosis (乾酪壞死) Signs and symptoms Most infections are restricted to the lungs Insidious at onset Non-specific : malaise, weight loss, cough, night sweats, sputum (purulent, bloody = lung tissue destruction, cavity) Primary focus is present in middle or lower lung fields. Hematogenous spread : disseminated (miliary粟粒狀) tuberculosis Clinical disease In most patients replication ceases within 3-6 wks after CMI is activated. PPD (+), x-ray (-) ~5% infected patients will progress to active disease within 2 years. PPD (+), x-ray (+) Other 5-10%, Latent PPD (+), x-ray (-), will develop disease sometime later in life. PPD (+), x-ray (+) Progression to disease is related to infectious dose and the patient’s immune competence. Pathogenesis No toxins and enzymes Histological signs (i.e., granuloma) are host immune responses to infection (DTH response) Immunopathology results in tissue necrosis (cytokine toxicity, complement activation, ischemia, etc.) Pathogenesis Small antigenic burden + protective immunity: activated macrophages can penetrate small granulomas (< 3 mm) and kill all bacteria with minimal tissue damage. But if many bacilli are present, cellular immune response (over-reactive, impaired) results in formation of large, necrotic or caseous granulomas encapsulated with fibrin, which protect bacteria from macrophage killing (latent), thus may be reactivated years later when patients’ immunologic responsiveness wanes. Epidemiology Humans - natural reservoir Non-human primates and guinea pigs experimental infection Person-to-person contact Inhalation: small particles containing one to three tubercle bacilli can reach alveolar spaces and establish infection. Patients with tubercle bacilli in sputum infect 10- 15 people/year. Ingestion of unpasteurized milk products (M. bovis) Epidemiology One third of the world’s population is infected. 9 million new cases and 2 million deaths annually. Endemic area: Southeast Asia, subSaharan Africa, Eastern Europe In USA, immigrants, homeless persons, drug and alcohol abusers, prisoners, HIV patients World TB incidence. Cases per 100,000; Red = >300, orange = 200–300; yellow = 100–200; green 50–100 and grey <50. Data from WHO, 2006. Clinical Diagnosis of M. tuberculosis In most patients, the only evidence of infection : • Radiographic evidence of calcification in lungs • Lifelong positive tuberculin skin test to PPD [Source: Wikipedia] • Lab detection of mycobacteria : microscopy or culture. Immunodiagnosis (for M. tuberculosis) Tuberculin skin test [Source: Wikipedia] • 5 unit PPD, intradermal; diameter of the area of induration after 48h • Patient’s response to exposure to M. tuberculosis (a DTH response) • Differentiate between infected and noninfected people. Reactivity to PPD 10 mm (positive): 3-4 wks after infection; lifelong acute disease or protective immunity = has been infected & develop immune response False negative: anergy (eg. HIV infection), overwhelming TB False positive: BCG vaccination, isoniazid treatment In vitro IFN-γ release assays Patient’s whole blood (contain sensitized T cells) Stimulate with M. tuberculosis-specific antigens (i.e., ESAT-6, CFP-10) Monitor IFN-γ production More sensitive and specific alternative to PPD skin test Microscopy The most rapid method to confirm mycobacterial disease acid-fast stain Carbolfuchsin strain : Ziehl-Neelsen method or Kinyoun method Auramine-rhodamine strain : Truant-fluorochrome method Specificity > 95% (mycolic acid) Sensitivity relate to (1) respiratory specimens and (2) specimens with high bacterial count Positive acid-fast stain = high infectivity Cannot identify mycobacterial species Sputum specimens with acid-fast stain “positive” : must differentiate between true infection with M. tuberculosis and transient colonization of other non-pathogenic spp. Culture Specimens: Pulmonary infection – abundant organisms in respiratory secretions Extrapulmonary infection – need repeated samplings Most isolates grow slowly; thus obscured by rapidly growing bacteria Specimen (sputum) decontaminated with 2% NaOH. Egg-based medium: Löwenstein-Jensen Agar-based medium: Middle-brook Broth culture of special formula : reduce culture time of most mycobacteria (from 34 wks to 10-14 days) Microscopic observation drug susceptibility assay (MODS): 24-well plate with broth and antimycobacterial drug Add specimen Mycobacteria growth is detected by an inverted microscope Susceptibility test done simultaneously 2. Mycobacterium leprae Leprosy (癩病) (Hansen’s disease): a chronic infection that affects skin and peripheral nerves (Schwann cells and macrophages) Since 1985, Global prevalence drops dramatically Endemic in few countries in Asia, Africa and Latin American Endemic in armadillos Nine-banded Armadillo (犰) Leprosy Long incubation period > 20 years Several clinical manifestations - depend on patient’s immune reaction to the bacilli Tuberculoid leprosy (類結核型痲瘋病) Lepromatous leprosy (痲瘋結節型痲瘋病) Tuberculoid leprosy Hypopigmented skin macula Peripheral nerve damage with complete sensory loss Lepromatous leprosy Many erythematous macula, papules or nodules Extensive disfiguring skin lesions (nasal cartilage, ears) Tuberculoid leprosy • Paucibacillary Hansen disease • Strong cell-mediated immunity • Granuloma in tissues with few bacilli; low infectivity Lepromatous leprosy • Multibacillary Hansen disease • Strong humoral immunity • Abundance bacilli in skin and peripheral nerves; infectious Transmission Person-to-person contact Inhalation of infectious aerosols or skin contact with respiratory secretions and wound exudates Diagnosis of M. lepare Base on clinical disease M. leprae cannot grow in cell-free cultures Histopathology - presence of acid-fast bacteria in lesions Skin test to lepromin Lepromin: derived from inactivated M. leprae Confirmative test for tuberculoid leprosy No use for lepromatous leprosy (anergy) 3. M. avium complex (MAC) Common environmental isolate in soil, water, plants. Developed after ingestion of contaminated food or water, no person-to-person spread In immunocompetent patients: M. avium subsp. intracellulare Recover from clinical specimens – mostly transient colonization (= infection, disease) Three forms: Middle-age or older men with a history of smoking and underlying pulmonary disease; Elderly, female nonsmokers (pneumonitis, bronchiectasis) Solitary pulmonary nodule In HIV-infected patients: • M. avium subsp. hominissuis • The most common mycobacterial disease in USA, typically disseminated • All organs are involved, especially in terminal stages with CD4 T counts < 10 cells/mm3 Tissue from a patient with AIDS who is infected with Mycbacterium avium complex 4. Slow-growing mycobacteria (3-8 weeks) Present in soil and water Opportunistic pathogens for immunocompromised patients Thus, isolation in specimens of immunocompetent patients mostly represents a transient colonization No person-to-person spread (except M. bovis) Slow-growing mycobacteria M. bovis M. kansasii Pulmonary tuberculosis M. scrofulaceum Lymphatic tissue infection M. ulcerans M. marinum M. haemophilum Cutaneous infection New species and spectrum of diseases are expending. 5. Rapidly growing mycobacteria (< 7 days) M. fortuitum, M. chelonae, M. abscessus. Relatively low virulence, susceptible to “conventional” antibacterial antibiotics Infections establish in deep subQ tissues after introduced by trauma or iatrogenic infections. No person-to-person spread Incidence increases as invasive procedures increases. Preliminary classification of mycobacteria by growth properties and colonial morphology 1. M. tuberculosis complex slow-growing, no pigmentation 2. Nontuberculous mycobacteria (NTM) Runyon group I slow-growing, yellow pigment (+) in light Runyon group II slow-growing, yellow pigment (+) in dark Runyon group III slow-growing, nonpigmented Runyon group IV rapidly growing A pigmented and / or a rapidly growing mycobacterium M. tuberculosis Preliminary identification Colonial morphological (pigmentation) and growth properties for preliminary identification is important. Only M. tuberculosis are transmitted from person to person. Thus only these patients are needed to be isolated and close contacts given prophylactic antibiotics. Guide empirical antimicrobial therapy Definitive identification Biochemical tests : production of nicain and reduction of nitrate (need > 3 weeks) Chromatographic analysis of cell wall lipids Species-specific nucleic acid probes for popular species For species without specific probe: amplification of species-specific 16S ribosomal RNA gene or SecA gene followed by sequence analysis Treatment (complex and controversial) Slow-growing mycobacteria: resistant to most common antibiotics, need multiple antimycobacterial agents for extended period (6-9 m) Isoniazid (INH) + rifampin + pyrazinamide + ethambutol for 2 months; then isoniazid + rifampin for 4 to 6 months Treatment Rapidly growing mycobacteria: resistant to commonly used antimycobacterial agents; susceptible to antibiotics - amikacin, imipenem, clarithromycin M. avium complex Clarithromycin + ethambutol + rifabutin M. leprae tuberculoid form: dapsone + rifampin for 6 m; lepromatous form: dapsone + rifampi + clofazimine for 12 m Effective of Treatment Right drug combination Sufficient treatment time Compliance Otherwise drug resistant strains may develop Multidrug-resistant M. tuberculosis (MDR-TB) : isoniazid and rifampin Extensively drug-resistant (XDR)-TB : drugs for MDR-TB + second line drugs (kanamycin, amikacin, capreomycin); untreatable Vaccination for M. tuberculosis Attenuated M. bovis (BCG strain) (France 1921) Effective in young people; less effective in adult Cannot be used for immunocompromised patients (i.e., HIV patients) Low skin test reactivity develops after vaccination – false postive IFN-γ release assays are not affected by BCG. Control Elimination is highly unlikely Active surveillance + prophylactic intervention + therapeutic intervention + case monitoring Directly Observed Treatment, Short Course (DOTS) 短期直接觀察療法 送藥到口 服藥入口 吃了再走 Chapter 27 Nocardia Gram (+), aerobic rod; form beaded filaments in tissues and cultures (similar to fungal hyphae). Contain mycolic acid (50-62 carbon), thus stain weakly acid-fast (use a weak decolorizing solution of hydrochloric acid). Epidemiology Ubiquitous presence, widespread in soil and in nature, > 100 species. Infections are exogenous. Cause disease in immuno-suppressed patients (AIDS, leukemia, transplant recipients) Transmission: unknown, no person-to person spread Pathogenesis Pathogenic strains Resist phagocytic killing Secrete catalase and superoxide dismutase Replicate in macrophages Cord factor (trehalose-6,6’-dimycolate): prevent phagosome-lysosome fusion Clinical disease - nocardiosis Lung and skin are primary sites of infections. Necrosis and abscess formation, sinus tract Highly predilection for hematogenous spread to CNS or skin Clinical diseases Bronchopulmonary infections: immunocompromised patient + pneumonia with cavitation + CNS or skin involvement Brain abscess : develop in 1/3 of patients, single or multiple Cutaneous infection: Primary (following trauma) or secondary (from a pulmonary site) Mycetoma, lymphocutaneous infections, skin infections with abscess and cellulitis Laboratory diagnosis Specimen: sputum, infected tissues Microscopic examination: Partially acid-fast, filamentous Culture: Medium for Legionella (BCYE agar) Aerial hyphae = hyphae protrude upward from the surface of a colony T/P/C Antibiotic – amikacin + cephalosporin + sulfonamides for > 6 wks Surgical debridement Localized infections – good prognosis Disseminated disease in immunocompromised patients – poor prognosis Actinomyces Greek words for “ray fungus” Form filamentous hyphae in specimens or culture G(+), anaerobic bacilli Contain no mycolic acid, non-acid-fast Pathogenesis normal flora of the mouth, alimentary tract and vagina (but not skin) low virulence potential, invade and cause disease when tissue is injured (trauma, surgery, or infection) unlike Nocardia, actinomyces are pathogenic to normal hosts, all infection are derived endogenously, no person-toperson spread. Actinomycosis Chronic granulomatous lesions Suppurative, abscess formation with sinus tracts Discharge (wound exudates) contain sulfur granules (pigmented microcolonies of organisms + calcium phosphate) Actinomycosis Cervicofacial (lumpy jaw) Poor oral hygiene, invasive dental procedure, oral trauma Patient with cervicofacial actinomycosis. Note the draining sinus tract. Actinomycosis Thoracic (lung): History of aspiration Abdominal: GI surgery, bowel trauma Pelvic: Intrauterine device user CNS: Solitary brain abscess, hematogenous spread Laboratory diagnosis Confirmation is difficult (normal population on mucosal surface) Sulfur granules for Gram’s stain Anaerobic culture: grow slowly, >2 wks. Colonies resemble top of a molar Treatment Surgical debridement of involved tissues Prolonged administration of antibiotics – penicillin, erythromycin, clindamycin Nocardia Actinomyces Mycolic acid 50-60 carbon No Acid-fast stain weak Negative Present Ubiquitous Normal flora Infection mode Exogenous Endogenous Host Immunosuppressed Colony Aerial hyphae Immunocompetent Resemble top of a molar