| South Alabama Medical Science Foundation
advertisement

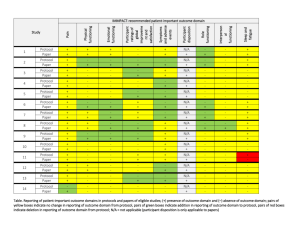
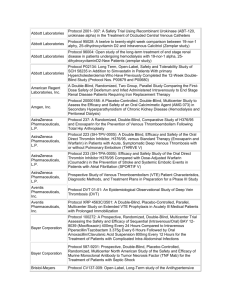
| South Alabama Medical Science Foundation | Cash Receipts (for Projects over $2,000 only) | October 1, 2014 - September 30, 2015 | | Project Dept | Number Internal Medicine Project Title Responsible Person Source of Funding 14-15 Receipts | 63690 A Randomized, Double-Blind, Parallel Group, Multicenter Phase IIIb Study to Compare Ticagrelor with Clopidogrel Treatment on the Risk of Cardiovascular Death, Myocardial Infarction and Ischaemic Stroke in Patients with Established Peripheral Artery Disease (EUCLID-Examining Use of tiCAgreLor In paD) Dr Awan, G Duke University 5,180.00 | 63707 High Resolution Optical Imaging of Esophageal Tissue Using the Nvision VLE Imaging System Registry Dr Cash, B NinePoint Medical 17,212.50 | 63714 A Multi-Center, Randomized, Open-Label, Controlled Study to Investigate the Treatment Response of Intraveneous Injectafer (Ferric Carboxymaltose) vs. Oral Iron to Baseline Hepcidin Levels in Patients with Iron Deficiency Anemin (IDA) Secondary to Inflammatory Bowel Disease (IBD) Dr Cash, B Luitpold Pharmaceuticals 14,250.00 | 63738 A Phase III Study Evaluating the Efficacy and Safety of Remimazolam (CNS 7056) Compared to Placebo and Midazolam in Patients Undergoing Colonoscopy Dr Cash, B PAION UK Ltd 3,000.00 | 63759 Functional Dyspepsia Reduction Evaluation and Safety Trial (FDREST): A Randomized, Double-Blind, Placebo-Controlled Study to Assess the Safety and Efficacy of Fdgard, a Medical Food, in the Dietary Management of Patients with Functional Dyspepsia Dr Cash, B IM Healthscience LLC 2,000.00 | 63603 Multi-center, Open-label, Extension Study to Evaluate the Long-term Efficacy and Safety of Oral Tolvaptan Tablet Regimen in Subjects with Autosomal Dominant Polycystic Kidney Disease (ADPKD) Dr Culpepper, M Otsuka Pharmaceutical 40,525.00 | 63717 TR02-A Randomized, Double-Blind, Placebo-Controlled, Parallel, 3-Arm Study of the Safety and Anti-Pruritic Efficacy of Nalbuphine HCL ER Tablets in Hemodialysis Patients with Uremic Pruritus Dr Culpepper, M Frenova, LLC 102,956.25 | South Alabama Medical Science Foundation | Cash Receipts (for Projects over $2,000 only) | October 1, 2014 - September 30, 2015 | | Project Dept | Number Project Title Responsible Person Source of Funding 14-15 Receipts | 63723 TR02Ext-An Open Label Extension Study of the Safety and Anti-Pruritic Efficacy of Nalbuphince HCL ER Tablets in Hemodialysis Patients with Uremic Pruritus. Dr Culpepper, M Frenova, LLC 39,112.50 | 63728 A Phase 3b, Multi-Center, Randomized-Withdrawal, Placebo-Controlled, Double-Blind, Parallel-Group Trial to Compare the Efficacy and Safety of Tolvaptan (45 to 120mg/day, Split-Dose) in Subjects with Chronic Kidney Disease Between Late Stage 2 to Early Stage 4 Due to Autsomal Polycysic Kidney Disease Dr Culpepper, M Otsuka Pharmaceuticals 4,500.00 | 63730 A Phase 3b, Multi-Center, Open-Label Trial to Evaluate the Long Term Safety of Titrated Immediate-Release Tolvaptan (OPC 41061, 30 mg to 120 mg/day, Split Dose) in Subjects with Autosomal Dominant Polycystic Kidney Disease Dr Culpepper, M Otsuka Pharmaceuticals 26,330.58 | 63739 A Phase 3, Multi-Center, Randomized, Open-Label, Active-Controlled Study of the Efficacy and Safety of FG-4592 in the Treatment of Anemia in Incident-Dialysis Patients Dr Culpepper, M Fibrogen, Inc 2,250.00 | 63743 A Phase 3,Open-Label, Randomized, Active-Controlled Study of the Efficacy and Safety of Roxadustat (FG-4592) in the Maintenance Treatment of Anemia in Subjects with End Stage Renal Disese (ESRD) on Stable Dialysis Dr Culpepper, M Fibrogen, Inc 2,250.00 | 63628 A Postmarketing Observational Study to Assess Respiratory Tract Adverse Events in Pulmonary Arterial Hypertension Patients Treated with Tyvaso (treprostinil) Inhalation Solution Dr Fagan, K United Therapeutics Corporation 5,937.50 | 63740 A Phase 2, Dose-Ranging, Randomized, Double-Blind, Placebo-Controlled Study of GS-4997 in Subjects with Pulmonary Arterial Hypertension Dr Fagan, K Gilead Sciences 2,500.00 | 63744 A Multi-Center, Double-Blind, Randomized, Placebo-Controlled, Phase 3 Study to Assess the Efficacy and Safety of Oral BPS-314d-MR Added-on to Treprostinil, Inhaled (Tyvaso) in Subjecs with Pulmonary Arterial Hypertension Dr Fagan, K Lung Biotechnology Inc 5,000.00 | South Alabama Medical Science Foundation | Cash Receipts (for Projects over $2,000 only) | October 1, 2014 - September 30, 2015 | | Project Dept | Number Project Title Responsible Person Source of Funding 14-15 Receipts | 62963 P05063-45, Long-Term Follow-Up of Subjects in a Phase 2 or 3 Clinical Trial in which SCH 503034 was Administered for the Treatment of Chronic Hepatis C Dr Herrera, J Schering-Plough Research Institute 7,517.00 | 63552 A Multicenter, Randomized, Double-Blind, Parallel Group, ActiveControlled Study to Evaluate the Efficacy and Safefy of Both Aliskiren Monotherapy and Aliskiren/Enalapril Combination Therapy Compared to Enalapril Monotherapy, on Morbidity and Mortality in Patients with Chronic Heart Failure (NYHA Class II-IV) Dr Massey, C Novartis Pharmaceuticals 5,893.39 | 63680 A Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Study to Evaluate the Effect of SAR236553/REGN727 on the Occurrence of Cardiovascular Events in Patients Who Have Recently Experienced an Acute Coronary Syndrome Dr Massey, C Sanofi US Services Inc 18,705.00 | 63753 An Open Label, Randomized Study to Determine the Rate of Cardiovascular Events at 1 Year for Patients with Elevated Troponins Post Major Non-Cardiac Surgery and the Impact of Ticagrelor Versus Aspirin on the Occurrence of Cardiovascular Events Named INTREPID: INvestigationg TicagRElor Treatement in Patients with Myocardial Injury Post non-carDiac Surgery Dr Omar, B The Cleveland Clinic Foundation 4,000.00 | 63696 BLI800-203 A Pilot Evaluation of an Experimental BLI800 Formulation for Bowel Preparation in Adult Patients Undergoing Colonoscopy Dr Rodriguez, R Braintree Laboratories 14,000.00 | 63758 BLI400-301 A Safety and Efficacy Evaluation of BLI400 Laxative in Constipated Adults Dr Rodriguez, R Braintree Laboratories 2,000.00 Total Internal Medicine $ 325,119.72 Surgery | 63669 Comparison of a Hemostatic Textile Dressing to Standard of Care for Control of Bleeding at Skin Graft Donor and Recipient Sites at Time of Surgical Grafting Dr Gulati, S Beeken Biomedical LLC 19,107.50 | South Alabama Medical Science Foundation | Cash Receipts (for Projects over $2,000 only) | October 1, 2014 - September 30, 2015 | | Project Dept | Number Project Title Responsible Person Source of Funding 14-15 Receipts | 63716 MEDIHONEY Versus Standard of Care for the Treatment and Outcome of Second Degree Superficial and Indeterminate Burns Dr Gulati, S Derma Sciences, Inc 3,278.00 | 63732 A Multicenter, Multinational, Randomized, Controlled, Blinded Study, Performed in Subjects with Thermal Burns to Evaluate the Efficacy and Safety of NexoBrid as Compared to Standard of Care (SOC) Treatment Dr Gulati, S MediWound Ltd 5,500.00 | 63677 Phase 3 Study of Efficacy and Safety of Topical E-101 Solution to Prevent Incisional Infections Among Colorectal Surgery Patients Dr Rider, P Excited States LLC 52,212.00 Total Surgery $ 80,097.50 Pediatrics | 62926 A Multicenter, Prospective, Long-term, Observational Registry of Pediatric Patients with Inflammatory Bowel Disease Dr Crissinger, K Centocor, Inc. 19,577.40 | 63661 A Phase 2 Multicenter, 36-Week Study to Assess the Safety and Effectiveness of Daily Oral Administration of Dexlansoprazole DelayedRelease Capsules for Healing of Erosive Esophagitis and Maintenance of Healed Erosive Esophagitis and Relief of Heartburn, in Adolescent Subjects Aged 12 to 17 Years Dr Crissinger, K Takeda Global Research & Development Center 4,333.10 | 63748 A Phase 3, Multicenter, Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Study to Evaluate the Efficacy and Safety of Ribipansel (GMI-1070) in the Treatment of Vaso-Occlusive Crisis in Hospitalized Subjecs with Sickle Cell Disease Dr Imran, H Pfizer 3,000.00 | 63722 A Multcentre, Randomised, Placebo-Controlled, Double-Blind Study of the Efficacy, Safety, and Pharmacokinetics of Lubiprostone in Paediatric Subjects Aged 6 or Greater to Less than or Equal to 18 Years with Functional Constipation Dr Ponnamballam, A Sucampo Pharma Americas, LLC 2,876.25 | South Alabama Medical Science Foundation | Cash Receipts (for Projects over $2,000 only) | October 1, 2014 - September 30, 2015 | | Project Dept | Number Project Title Responsible Person Source of Funding UAB 11,000.00 Mast Therapeutics Inc 31,030.00 Selexys Pharmaceuticals 14,487.80 | 63726 SAMSF/UAB : Healthy Active Living Sessions with ACHIA Dr Preud'Homme, D | 63678 Evaluation of Purified Poloxamer 188 in Children in Crisis (EPIC): A Phase 3 Randomized, Double-Blind, Placebo-Controlled, Multicenter Clinical Trial of ANX-188 (purified poloxamer 188) Injection in Children with Sickle Cell Disease Experiencing Vaso-Occlusive Crisis Dr Siddiqui, A | 63720 A Phase II, Multicenter, Randomized, Placebo-Controlled, Double-Blind, 12-Month Study to Assess Safety and Efficacy of SelG1 with or without Hydroxyurea Therapy in Sickle Cell Disease Patients with Sickle-Cell Related Pain Crises Dr Wilson, F Total Pediatrics 14-15 Receipts $ 86,304.55 OBGYN | 63646 A Clinical Field Study to Evaluate the Clinical Performance of the SEQureDx Trisonomy 21 Test in the Detection of the Relative Quantity of Chromosome 21 in Circulating Cell Free DNA extracted from Maternal Blood Plasma Obtained from Pregnant Women with High Risk Indicatiors for Fetal Chromosome 21 Aneuploidy Dr Lewis, D Sequenom 7,437.50 | 63719 SQNM-T21-107 - Collection of Whole Blood Speciment from Pregnant Women at Increased Risk for Fetal Chromosomal Abnormality for Use in Development of a Noninvasive Prenatal Test in the Detection of the Relative Quantity of Chromosomal Material in Circulating Cell-Free DNA Extracted from Maternal Plasma Dr Lewis, D Sequenom 20,500.00 | 63734 Prospective Randomized Double-Blind, Placebo-Controlled Evaluation of the Pharmokinetics, Safety and Efficacy of Recombinant Antithrombin Versus Placebo in Preterm Preeclampsia (PRESERVE-1), Protocol no. RB AT PPE 01-13 Dr Lewis, D rEVO Biologics 106,698.75 Total OBGYN $ 134,636.25 | South Alabama Medical Science Foundation | Cash Receipts (for Projects over $2,000 only) | October 1, 2014 - September 30, 2015 | | Project Dept | Number Project Title Responsible Person Source of Funding 14-15 Receipts USAMCI | 63504 An International Multi-Centre Open-Label 2-Arm Phase III Trial of Adjuvant Bevacizumab in Triple Negative Breast Cancer, Protocol No. BO20289 and Roche Sample Repository Research Projec, Protocol No. B020289RG Dr Butler, T Hoffman-La Roche, Inc 10,231.00 | 63560 A Randomized, Phase III, Double-Blind, Placebo-Controlled Multicenter Trial of Everolimus in Combination with Trastuzumab and Paclitaxel, as First Line Therapy in Women with HER2 Positive Locally Advanced or Metastatic Breast Cancer Dr Butler, T Novartis Pharmaceuticals 31,048.57 | 63718 A Multicenter Phase 3 Randomized, Open-Label Study of Bosutinib Versus Imatinib in Adult Patients with Newly Diagnosed Chronic Phase Chronic Myelogenous Leukemia Dr Butler, T Avillion Development 5,000.00 | 63685 A Phase 3, Randomized, Open Label Trial of Lenalidomide/Dexamethasone with or without Elotuzumab in Subjects with Previously Untreated Multiple Myeloma Dr Clarkson, D Bristol Myers Squibb 20,511.00 | 63715 A Randomized, Double-Blind, Multicenter, Parallel-Group, Phase III Study to Evaluate Efficacy and Safety of DCV AC/Pca versus Placebo in Men with Metastatic Castration Resistant Prostate Cancer Eligible for 1 st Line Chemotherapy Dr Clarkson, D SOTIO 6,500.00 | 63745 A Phase 3, Double-Blind Randomized Study to Compare the Efficacy and Safety of Rituxlmab Plus Lenalldomide (CC-5013) Versus Rituxlmab Plus Placebo in Subjects with Relapsed/Refractory Indolent Lymphoma Dr Clarkson, D Celgene Corporation 6,500.00 | 63746 Multicentre, Randomised, Double-Blind, Phase III Trial to Investigate the Efficacy and Safety of Oral Nintedanib Plus Docetaxel Therapy Compared to Placebo Plus Docetaxel Therapy in Patients with Stage IIIB/IV or Recurrent, Adenocarcinoma Subtype Non-Small Cell Lung Cancer After Failure of First Line Chemotherapy Dr Clarkson, D Boehringer Ingelheim Pharmaceuticals 6,000.00 | South Alabama Medical Science Foundation | Cash Receipts (for Projects over $2,000 only) | October 1, 2014 - September 30, 2015 | | Project Dept | Number Project Title Responsible Person Source of Funding 14-15 Receipts | 63749 A Phase 3 Study Comparing Daratumumab, Lenalidomide, and Dexamethasone (DRd) vs Lenalidomide and Dexamethasone (Rd) in Subjects with Previously Untreated Multiple Myeloma Who Are Ineligible for High Dose Therapy Dr Clarkson, D Janssen Research & Development 6,000.00 | 63757 A Phase 3, Randomized, Study to Assess the Efficacy and Safety of Ubiituxlmab in Combination with Ibrutinib Compared to Ibrutinib Alone, in Patients with Previously Treated High-Risk Chronic Lymphocytic Leukemia (CLL) Dr Clarkson, D TG Therapeutics, Inc 6,500.00 | 63710 Multi Institutional Neo-Adjuvant Therapy Mammaprint Project (MINT) Dr Dyess, L Agendia, Inc 2,400.00 | 63689 A Registry of Caris Life Sciences Molecular Intelligence Service (Biomarker Assessment Results) Intended for Correlation with Cancer Clinical Outcomes Dr Finan, M Caris 7,700.00 | 63666 A Phase III Randomized, Double Blind, Placebo-Controlled Study of BKM120 with Fulvestrant, in Postmenopausal Women with Hormone Receptor-Positive HER2-Negative Locally Advanced or Metastatic Breast Cancer Which Progressed on or after Aromatase Inhibitor Treatment Dr Norden, C Novartis Pharmaceuticals 17,681.35 | 63701 An Observational Cohort Study of Treatment Patterns and Outcomes in Patients with HER2 Positive (HER2+) Metastatic Breast Cancer Dr Norden, C Genentech 7,896.14 | 63721 A Multicenter, Multinational, Phase II Study to Evaluate Pertuzumab in Combination with Trastuzumab and Standard Neoadjuvant AnthracyclineBased Chemotherapy in Patients with HER2-Positive, Locally Advanced, Inflammatory, or Early-Stage Breast Cancer Dr Norden, C Genentech Inc 58,052.70 | 63735 A Randomized Multicenter Pivotal Study of CDX-011 (CR011-vcMMAE) in Patients with Metastatic, GPNMB Over-Expressing, Triple-Negative Breast Cancer Dr Norden, C Celldex Therapeutics 7,000.00 | South Alabama Medical Science Foundation | Cash Receipts (for Projects over $2,000 only) | October 1, 2014 - September 30, 2015 | | Project Dept | Number | 63673 Project Title A Phase III, Randomized, Double-Blind, Active Comparator-Controlled Parallel-Group Study, Conducted Under In-House Blinding Conditions, to Examine the Efficacy and Safety of a Single 150mg Dose of Intravenous Fosaprepitant Dimeglumine for the Prevention of Chemotherapy-Induced Nausea and Vomiting (CINV Associated with Moderately Emetogenic Chemotherapy Responsible Person Source of Funding 14-15 Receipts Dr Rocconi, R Merck 8,494.00 | 63725 Modular Phase II Study to Link Targeted Therapy to Patients with Pathway Activated Tumors: Module 3 - MEK 162 for Patients with RAS/RAF/MEK Activated Tumors Dr Rocconi, R Novartis Pharmaceuticals 7,500.00 | 63752 Double-Blind, Placebo Controlled Phase III Trial of Maintenance FANG (bi-shRNA and GMCSF Augmented Autologous Tumor Immunotherapy) for High Risk Stage III/IV Ovarian Cancer Dr Rocconi, R Gradalis, Inc 5,000.00 | 63755 A Phase I, Open-Label, Multiple-Ascending Dose Trial to Investigate the Safety, Tolerability, Pharmokinetics, Biological and Clinical Activity of MSB0010718C in Subjects with Metastatic or Locally Advanced Solid Tumors and Expansion to Selected Indications Dr Rocconi, R EMD Serono, Inc 6,500.00 | 63731 A Phase 3b, Multicenter, Open-Label, Single-Arm, Expanded Access Protocol of Talimogene Laherparepvec for the Treatment of Subjects with Unresected, Stage IIIB to IVM1a Melanoma Dr Tan, M Amgen 12,268.00 | 63682 STEAM (Sequencing Triplet with Avastin and Maintenance) : FOLFOXIRI/Bevacizumab Regiments (Concurrent and Sequential) vs. FOLFOX/Bevacizumab in First-Line Metastatic Colorectal Cancer Dr Taylor, W Genentech 21,117.33 | 63693 Prospective International Observational Cohort Non-Comparative Study Describing the Safety and Efficacy of ZALTRAP Administered in Combination with FOLFIRI for the Treatment of Patients with Metastatic Colorectal Cancer in Current Clinical Practice: A Post-Authorisation Safety Study (PASS) Dr Taylor, W Sanofi US Services Inc 4,675.00 | South Alabama Medical Science Foundation | Cash Receipts (for Projects over $2,000 only) | October 1, 2014 - September 30, 2015 | | Project Dept | Number Project Title Responsible Person | 63742 A Phase 2, Randomized, Multicenter Study of PEGPH20 (PEGylated Recombinant Human Hyaluronidase) Combined with nab-Paclitaxel Plus Gemcitabine Compared with nab-Paclitaxel Plus Gemcitabine in Subjects with Stage IV Previously Untreated Pancreatic Cancer Dr Taylor, W | 63724 A Randomized, Open-Label, Multicenter, Phase 3 Trial Comparing Vellparib Plus Carboplatin and Paclitaxel Versus Investigator's Choice of Standard Chemotherapy in Subjects Receiving First Cytotoxic Chemotherapy for Metastatic or Advanced Non-Squamous Non-Small Cell Lung Cancer (NSCLC) and Who Are Current or Former Smokers Dr Vu, M Source of Funding 14-15 Receipts Halozyme, Inc 7,500.00 AbbVie Inc 6,075.00 Total USAMCI $ 278,150.09 Total All Departments $ 904,308.11 Other Research Related Revenue: Royalties & Licensing Fees Ayling/Bailey Merck Eprova Ayling/Bailey Merck Eprova- Reimb for Legal 187,407.90 Intellect USA Licensing 32,500.00 QED 5,960.00 Pappolla/Poegller Various 2,380,353.00 Total Other Research $ 2,606,220.90 Total $ 3,510,529.01