Medicines Optimisation in IBD
Can we base it on evidence?
Anja St.Clair Jones
Lead Pharmacist Digestive Diseases
Royal Sussex County Hospital
Brighton
Aims and Objectives
Enable medicines optimisation in IBD
• Understand Inflammatory Bowel Disease
(IBD),
• Describe drugs used in treatment of IBD
• Develop strategies for medicines
optimisation
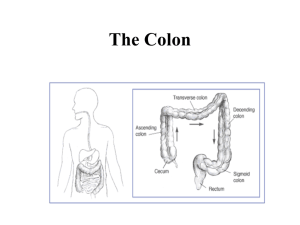
Stomach
Duodenum
Splenic flexure
Hepatic flexure
Transverse colon
Descending colon
(Left sided/distal)
Jejunum
Ascending colon
(Right sided/
proximal)
Ileum
Caecum
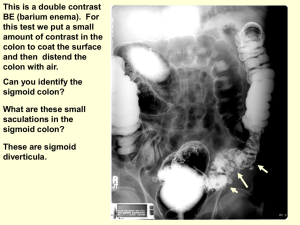
Sigmoid colon
Terminal ileum
Rectum
Anus
Epidemiology
• Disease of YOUNG people (peak 10-25y, 50+y)
• Up to 260’000 people affected in UK
• UC: 10/100’000 per year
–
–
–
–
–
–
prevalence 146/100’000 (NICE 2013, CG 166)
Incidence stable
Difference in ethnic groups (Ashkenazi Jews)
50% have relapse in any year
25% acute sever colitis during lifetime (NICE 2013)
90% are able to FT work 1year after diagnosis
• CD: 5-10/100’00 per year
–
–
–
–
–
prevalence 157/100’000 (NICE 2012, CG152)
Incidence increasing
75% able to work in year after diagnosis
15-20% disabled by disease within 5 years (NICE 2012)
50-80% require surgery for strictures (NICE 2012)
Anatomic distribution in
Crohn’s
80% left sided
only
Pathogenesis
Theories of inflammatory bowel disease etiology
-Toxic response to luminal contents
-Specific microbial pathogen
-Abnormal luminal constituents
-Increased absorption of luminal macromolecules
-Enhanced immunologic response to normal constituents
-Autoimmune response
-To epithelial cell or mucus glycoproteins
-Molecular mimicry (cross-reactivity of intestinal microflora and
epithelia)
-To immune cells
Trigger – what?
Genetic involvement
Immune dysregulation in
Crohn's disease
CD or UC?
Ulcerative colitis
Crohn's disease
Colon only
Any part of gastrointestinal
tract
Bleeding
+++
+
Diarrhea
+++
++
Abdominal pain
++
+++
Growth failure
+
+++
Tenesmus
+++
±
Perianal disease
—
+
Rectal involvement
+++
+
Inflammation
Continuous
Discontinuous
Diffuse erythema
Patchy lesions
Ulceration in inflamed mucosa
Discrete ulcers in normal
mucosa
Exceedingly uncommon
Frequent
Site of disease
Symptoms
Endoscopic findings
Complications
Fistulas
(? Crohn's)
Strictures
Uncommon (? malignancy)
Common
Cancer risk (>10 years)
Increased
Increased
Fistulae in IBD
Diagnosis and investigations
•
•
•
•
•
•
•
•
History and examination
FBC, LFT, ESR or CRP
Microbiological testing (C. Diff., CMV)
Abdo imaging
Endoscopies +/- biopsies
Barium enema, small bowel studies
Colonoscopy
Assessment of disease extent
Figure 1a and 1b: endoscopic views of Crohn’s disease
showing mucosal oedema, ulceration and exudates.
Crohn's Disease Activity Index
•
•
•
•
CDAI = 2x1 + 5x2 + 7x3 + 20x4 + 30x5 + 10x6 + 6x7 + (weight factor)8
1. Number of liquid or very soft stools in one week
2. Sum of seven daily abdominal pain ratings:
(0=none, 1=mild, 2=moderate, 3=severe)
3. Sum of seven daily ratings of general well-being:
(0=well, 1=slightly below par, 2=poor, 3=very poor, 4=terrible)
4. Symptoms or findings presumed related to Crohn's disease
arthritis or arthralgia
iritis or uveitis
erythema nodosum, pyoderma gangrenosum, apththous stomatitis
anal fissure, fistula or perirectal abscess
other bowel-related fistula
febrile (fever) episode over 100 degrees during past week
5. Taking Lomotil or opiates for diarrhea
6. Abnormal mass
0=none; 0.4=questionable; 1=present
7. Hematocrit [ (Typical - Current) x 6 ]
•
8. 100 x [(standard weight-actual body weight) / standard weight]
•
•
•
•
Harvey–Bradshaw Index for Crohn's
disease
• Number of liquid stools per day
• Abdominal pain, sum of seven daily ratings:
(0-none, 1-mild, 2-moderate, 3-severe)
• Abdominal mass
(0-none, 1-questionable, 2-definite, 3-definite & tender)
• General well being
(0-very well, 1-slightly below par, 2-poor, 3-very poor, 4-terrible)
• Complications (score 1 point per item)
Arthritis/arthalgia
Skin/mouth lesions
Iritis/uveitis
Anal fissure, fistula/perianal abscess
UC activity scores
Therapeutic aim
• Remission
• Avoid surgery
• CRC (5x)
Also:
– Smoking cessation
– VTE prophylaxis (always!!)
– Pain control (no NSAIDs)
– Osteoporosis prophylaxis
– Opportunistic infections
–
https://www.ecco-ibd.eu/documents/ECCOconsensusOI.pdf
Treatments
How to optimise treatment?
•
•
•
•
•
Correct dose
Co-prescribing
TDM
Exit strategies
Rescue strategies
Steroids
• Indication: CD and UC
– Moderate to severe relapse
• Maximise local effect and limit systemic effect
• No role in maintenance
– 40mg OD Prednisolone reduced slowly by 5mg/week
– ≤ 15mg ineffective in active disease
• Budesonide not as effective as Pred but
alternative in ileo-ascending colonic disease
– Less systemic effect
• Osteoporosis
Rectal steroids
Only for patient not responding to rectal mesalazine
• Hydrocortisone (Colifoam) 1 od-bd
– High plasma levels after administration
• Prednisolone NaPhos (Predsol) 1 bd
– Rectal mucosa only
• Prednisolone metosulphbenzoate (Predenema 1 od)
– Poorly absorbed
– Increased spread (reached ascending colon in some patient)
• Prednisolone metosulphbenzoate (Predfoam 1od-bd)
– Poorly absorbed
– Retained in rectum and sigmoid colon
Rectal steroids
Rectal steroid
Peak plasma nmols/L
Peak tissue ng/g
Prednisolone
phosphate 20mg
365 (2hrs)
44
Predenema
Prednisolone metasulphbenzoate 20mg
45 (2hrs)
257
Predfoam
Prednisolone metasulphbenzoate 20mg
320 (4hrs)
4874
Colifoam
Hydrocortisone
Acetate 125mg
510(4hrs)
Not recorded
Optimisation
• Correct dose
– Start at 40mg and slow reduction
• Correct formulation
– Know where the disease is located
• Prevent osteoporosis
• Consider infection risk
Rectal reparations: site of
action and indication
Formulation
Site of action
Disease extent
Suppository
Rectum
Proctitis
Foam
Sigmoid Colon
Procto-sigmoiditis
Enema
Descending colon to splenic
flexure and in some cases
even distal part of transverse
colon.
Left sided ( distal)colitis
• Dose:
• Crohn’s:
5-ASA
– higher doses ≥ 4g no evidence (post op only)
• UC:
– Induction of remissions ≥ 4g/day
– Maintenance of remission ≥ 2g/day
• Rectal preparations (PINCE)
– 15% past splenic flexure:2g bd oral + 1g OD
rectal (64% remission at week 8 vs 43% oral)
• Compliance at week 8
– (PODIUM: OD vs BD:71% vs 59% remission)
Drug
Mesalazine
Asacol MR
Ipocol
Mesren MR
Octasa MR
Mezavant XL
Formulation
Optimal
release pH
400mg: Enteric coated with Eudragit S
800mg: Enteric coated with layer of Eudragit S
followed by Eudragit S+L
Enteric coated with Eudragit S
Enteric coated with Eudragit S
Enteric coated with Eudragit S
Film coated with methacrylate copolymers Type
A, Type B
Pentasa
Ethylcellulose coated microgranules to allow
slow continuous release
Salofalk
Tablets: Enteric coated with Eudragit L
Granules: Eudragit L and matrix
structure (slow continuous release)
Azo-bonded preparations
Salazopyrin
5-ASA +SA
(Sulfasalazine)
Colazide
Prodrug
(Balsalazide)
Dipentum
Dimer
(Olsalazide)
granule
drug Site of drug release
pH-dep. delayed
release (>7)
Terminal ileum & large bowel
(colon & rectum)
>7
>7
>7
Gastroresistant
coating with
Lipophylic and
hydrophilic matrix
(>7)
Terminal ileum & colon
Terminal ileum & colon
Terminal ileum & colon
Colon
Diffusion through
semi-permeable
membrane (Enteral
pH)
pH-dep. delayed
release (>6) and
matrix
Duodenum to rectum
Terminal ileum & colon
Cleavage by intestinal Colon
bacteria
Azoreductase
(>7)
Adherence and switching
• 39% adherence in maintenance
Robinson; APT 2013
– 61% chance of relapse vs 11%
– Increased risk of CRC 31% vs 3%
• 75% risk reduction in adherers
– Cost :14% admission = 49% of cost
Kane 2006, Bassi 2004, Hawthorne 2008
• Switch patients had 3.5-fold risk of relapse
• Endoscopic healing rate is not equivalent
Optimisation
• Top and tail in sever flares
• Consider switching carefully
• Support Adherence
– Tailor formulation to patient
– Reinforce message of CRC prevention
– Consider switch of preparation carefully
– Consider impact on endoscopic healing