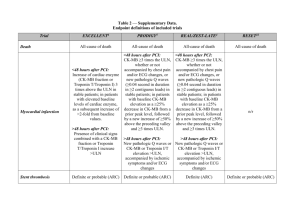
SUPPORT-1
advertisement
A Prospective Randomized Trial of CMX2043, a Lipoic Acid-Based Cytoprotectant, In Patients Undergoing Elective PCI: Primary Results of the SUPPORT-1 Trial Mitchell W. Krucoff, C.K. Ponde, Jagdish Hiremath, Mullasari Ajit, Eddison Ramsaran, Manesh R. Patel, Alan S. Lader, F. Howard Schneider, Reinier Beeuwkes, C. Michael Gibson, and James E. Tcheng. 20090417 Conflict of Interest Clinical Advisory Board, Ischemix 20090417 Incidence & Implications of Peri-Procedural MI: A Controversy of Definitions & Pathophysiology Incidence: 3.6-17.8% Resolute All Comers 2,121 patients 3 yr mortality: 2-8% 20090417 PCI: A Human Laboratory for Cytoprotection PCI produces enzymatic events Selected protein elevations (CPK-MB, Troponin) represent myocellular necrosis PCI as a laboratory for RCT of cytoprotective strategies has FDA predicate (vitamin B6 metabolite pyridoxal-5’phosphate monohydrate (MC1) in the MC-1 to Eliminate Necrosis and Damage (MEND-1) 20090417 CMS-2043: A Novel Molecular Entity to Inhibit Ischemic Apoptosis • Reactive oxygen species (ROS) anti-oxidant, AND • Activates Akt (Ak mouse thymoma = Protein kinase B) via tyrosine kinase (TK) 20090417 Safety and Efficacy of CMX-2043 in Subjects Undergoing PCI and PeriOperative Reperfusion Treatment The SUPPORT 1 Study 20090417 SUPPORT-1: Study Design Phase IIa Safety & Efficacy: CMX-2043 Prospective, randomized 3:1 • 3 doses (0.8, 1.6 & 2.4 mg/kg) vs placebo Multi-center (N=6) Elective PCI Patients • WNL biomarkers & Non-acute ECG • Receiving single stent of ≥ 18 mm or multiple stents Primary Outcome Measures: • Incidence of CK-MB elevation <24 hours following PCI • Change in cardiac biomarkers <24 hrs following PCI CK-MB, Troponin T Secondary Outcome Measure: MI as >X3 peak CPK 20090417 SUPPORT-1 Exclusion Criteria Acute/unstable angina MI within 14 days Coagulopathy Clinical valvular disease Clinical peripheral vascular disease TIA, stroke or IC bleed within 90 days Creatinine level ≥ 1.5 times ULN 20090417 SUPPORT-1 Sites and Investigators 20090417 SUPPORT I: Patient Accrual/Randomization Total patients enrolled N=142 0.8 mg/Kg N= 36 1.6 mg/Kg N= 35 2.4 mg/Kg N= 36 Placebo N= 35 0.8 mg/Kg (100%) 1.6 mg/Kg (97.1 %) 2.4 mg/Kg (100%) Placebo (100%) 1 subject withdrawn 20090417 SUPPORT-1 Baseline Characteristics 20090417 SUPPORT I: Arteries Stented Per Rx Group 20090417 Primary Endpoint: 24 Hr CK-MB Change from Baseline 20090417 24 Hr CK-MB Change From Baseline (ng/mL) p = 0.05 p=0.05 vs. Placebo CMX-2043 treatment 20090417 24 Hr Troponin T Change from Baseline CMX-2043 treatment 20090417 24 Hr Peri-Procedural MI by CK-MB >X3 ULN p=0.024 vs. Placebo CMX-2043 treatment 20090417 24 Hr Peri-Procedural MI by Troponin T >X3 ULN p=0.050 vs. Placebo CMX-2043 treatment 20090417 SUPPORT-1 Adverse Events Summary 0.8 mg/kg 1.6 mg/kg 2.4 mg/kg Placebo (N = 36) (N = 35) (N = 36) (N = 34) Any AEs 7 ( 19.44%) 14 ( 40.00%) 11 ( 30.56%) 10 ( 29.41%) Drug Related AEs 0 ( 0.00%) 3 ( 8.57%) 1 ( 2.78%) 2 ( 5.88%) Serious AEs 1 ( 2.78%) 2 ( 5.71%) 1 ( 2.78%) 0 ( 0.00%) AEs leading to Study discontinuation 0 ( 0.00%) 1 ( 2.86%) 0 ( 0.00%) 0 ( 0.00%) Deaths 0 ( 0.00%) 0 ( 0.00%) 0 ( 0.00%) 0 ( 0.00%) Category 20090417 SUPPORT I: Limitations Serum marker elevations with elective PCI have biochemical relevance for NME testing vs. human apoptosis, however the clinical relevance of these findings is unproven 20090417 SUPPORT I Primary Results: Conclusions SUPPORT I was a prospective, randomized, multicenter Phase IIa dosing study of protection from PCI-induced myonecrosis by CMX-2043 infusion All doses of CMX-2043 studied (0.8, 1.6 and 2.4 mg/kg) appeared safe in this population High dose (2.4 mg/kg) infusion of CMX-2043 was associated with statistically significant reduction of serum markers of myonecrosis and MI defined by >3X elevation above ULN Results of SUPPORT I suggest the basis for further study and a Phase III study design 20090417