03-Intracellular Accumulations
advertisement

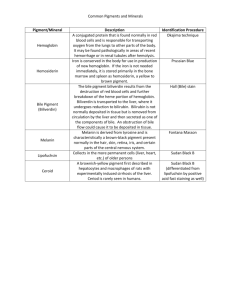
General Pathology (PATH 303) Lecture # 6 INTRACELLULAR ACCUMULATIONS Steatosis (fatty change, lipidosis); it is abnormal accumulation of neutral fats (triglycerides) within parenchymal cells. More severe than hydropic degeneration. Most common in liver, heart, kidney, and skeletal muscles. Causes: 1. hypoxia (Anoxic anoxia) 2. Hepatoxins-bacterial, plant –chemical poisons. 3. Metabolic diseases e.g. diabetes, ketosis 4. Deficiency of lipotropic substances e.g. choline. 5. Miscellaneous – starvation, obesity. Pathogenesis: Several mechanisms e.g. Excessive mobilization of fat and entry into the cell. Decreased fatty acid oxidation as in hypoxia. Decreased synthesis of apoprotiens. Impaired lipoprotien secretion from liver Gross appearance: Severe form: increase in size, friable and rupture of organs. Change in color, cats-white, cattle- yellow, horsesorange. Cut surface buldges and capsule retracts. Heart muscle has thrush breast appearance. Microscopic appearance: Early, mild condition- small membrane bound inclusions (liposomes) near ER. Appear as clear vacuoles which fuse & become large and displace the nucleus towards periphery. Special stains Oil red-O and Sudan IV Significance: Reversible change If injury persist, may cause death of cell Obesity: excessive accumulation of adipose tissue in the fat depots. Excessive intake of fat and Carbohydrates.May cause sterility, diabetes and atherosclerosis 1. 2. 3. 4. 5. Glycogen: Abnormal accumulation occurs in liver, muscles and kidney in case of: Diabetes mellitus ( hyperglycemia) Corticosteroid therapy Hereditary glycogen storage diseases. Neutrophil leukocytes in inflammatory conditions. Certain neoplasms Gross and microscopic appearance: Affected organ becomes enlarged and pale. Glycogen appears as clear vacuoles in smooth endoplasmic reticulum. Special stain - iodine gives reddish brown color and Best’s carmine stains it red. Tissues should be fixed in absolute alcohol. Protein inclusions: Protein inclusions or inclusion bodies have different size, shape, staining reaction and location in the cell. Identification of inclusions used in diagnosis of several diseases. Location: may be intracytoplasmic or intranuclear or both. Shape; may be spherical, globular or homogenous. Staining affinity: may be eosinophilic or basophilic. Mechanism of inclusion formation: viral infection, toxic injury, absorption of external proteins and secretory stasis. Absorption inclusions: Seen in renal epithelial cells in albumen- uria-- endocytic uptake- seen in proximal convoluted tubules. Protein sequestered in phagolysosomes. Appear as hyaline droplets Secretory inclusion: Secretory synthesis exceeds the capacity for excretion from the cell. Commonly seen in liver- composed of albumen and fibrinogen. Plasma cells develop cytoplasmic condensed globular inclusions. Pigment disorders: Pigments are colored substances, synthesized within the body (endogenous) or coming from outside (exogenous). Endogenous pigments: include melanin, lipofuscin and derivatives of hemoglobin Melanin: A brownish black pigment formed by oxidation of tyrosine to dihydroxyphenylalanine by enzyme tyrosinase Melanocytes , the cells which produce melanin are of neuroectodermal origin, present in the basal layer of epidermis. Black, brown, red color of skin due to amount and distribution of melanin. Protects against UV rays in sunlight. Microscopic appearance: melanin granules are small, uniform, dirty brown, round granules. Foci of melanocytes may be located in intestine, heart, kidney etc called melanosis – usually harmless. Hormonal disturbances may cause hyperpigmentation – acanthosis nigricans – observed in dogs due to lesions in adrenal. Pathological amounts – associated with tumors of melanocytes, melanomas and melanocarcinomas – common in gray horses. Nevus is a small accumulation of melanocytes in the skin. Albinism: absence of melanin. Such individuals have melanocytes but are unable to synthesize melanin due to lack of tyrosinase. These patients are vulnerable to cancer. Leukoderma is local loss of pigment observed in areas with rubbing injuries from collar, saddle, harness etc. Lipofuscin : it an insoluble brownish yellow pigment also called lipochrome, wear and tear pigment or ageing pigment. It contains complexes of lipid and protein derived from peroxidation of lipids by free radicals. It represents indigestible residues of autophagic vacuoles . It gives brown discolorations to tissues but is not injurious to the cell. Derivatives of hemoglobin: Destruction of old ( senescent) erythrocytes occurs within mononuclear phagocytic cells of spleen, liver and bone marrow and Hb is released. Break down of Hb gives rise to globin (protein) and pigment complex heme. Globin is soluble and is removed by blood and lymph. Oxidation of heme by heme oxygenase gives rise to biliverdin, carbon monoxide and iron. Body iron occurs in two forms – functional (80%) in Hb, myoglobin and iron containing enzymes catalase and cytochrome.The storage pool includes 15-20% of total body iron in the form of ferritin and hemosiderin. Ferritin: it is a protein – iron complex found in liver, spleen, bone marrow and skeletal muscles. When there is excess of iron , ferritin forms hemosiderin. A. hemosiderin : it is a golden- yellow, granular pigment which is a storage form of iron. Occurs in localized or systemic accumulations. 1. localized haemosiderosis : This occurs in local injuries, haemorrhages, bruises, hematomas etc. Grossly the bruise goes through different changes indicating formation of different pigments as below: 1-Red blue – Hb 2. Greenish blue ( biliverdin green ) 3. Pinkish blue ( bile pigment ) and 4. Golden yellow – hemosiderin, observed in scars. Systemic hemosiderosis: In this case hemosiderin is deposited in many organs and tissues, especially in the liver, spleen, lymph node, bone marrow etc. It is caused by increased dietary iron, impaired utilization, hemolytic anemia and transfusion Gross appearance of hemosiderin: Hemosiderin appear as golden yellow, granular pigment in the cytoplasm of macrophages. It is seen in renal epithelial cells in intravascular hemolysis in equine infectious anemia. In the lungs hemosiderin occurs in chronic passive congestion. RBC’s released in the lung alveoli are engulfed by alveolar macrophages. Hemosiderin laden macrophages are known as heart failure cells and the condition is cell brown induration of lung. Prussian blue reaction is used for identification of haemosiderin. Potassium Ferrocyanide reacts with ferric iron and converts it into insoluble blue black ferric ferrocyanide. B. Bilirubin : Bilirubin is the major pigment of bile. Accumulation of bilirubin in blood (hyperbilirubinemia) causes its deposition in the tissues and the clinical condition is called jaundice. Formation of bilirubin: There are different types of bilirubin and its formation goes through following stages 1. Hemobilirubin formation 2. Transport to blood. 3. Uptake and intracellular transport 4. Glucuronidation 5. Secretion into the bile canaliculi. Oxidation of heme by heme oxygenase produces biliverdin (green pigment) CO and iron. Iron is sequestered in ferritin and hemosiderin. Biliverdin is reduced by biliverdin reductase into yellow pigment bilirubin inside the macrophages. Bilirubin is bound with albumen and transported to blood where it circulates as hemobilirubin or unconjugated bilirubin. It is insoluble in water and does not pass through the renal filter. Hemobilirubin is taken up by the hepatocytes and transported to ER where it is conjugated to glucuronide as conjugated bilirubin. Conjugated bilirubin ( bilirubin diglucuronide ) is soluble in water and is not toxic, excreted into canaliculi. Conjugated bilirubin reaches intestine where glucuronide is separated and bilirubin is converted into urobilinogen by bacteria. Most of urobilinogen is excreted into feces and about 20% is reabsorbed and returned to liver for re-excretion into bile. A small amount reaches kidneys and is excreted into urine. Jaundice IT IS THE INCREASE OF BILIRUBIN IN THE BLOOD AND ITS DEPOSITION IN THE TISSUES LIKE SKIN AND SCLERA GIVING THEM YELLOW DISCLOURATION. ACCORDING TO THE TYPE OF BILIRUBIN AND ITS DISTRIBUTION JAUNDICE MAY BE HEMOLYTIC (PREHEPATIC), TOXIC (INTRAHEPATIC) OR OBSTRUCTIVE (POSTHEPATIC) TYPES 1-hemolytic(prehepatic jaundice) More than 80% serum bilirubin is unconjugated (hemolytic bilirubin). There are three mechanisms A. overproduction of hemobilirubin due to intravascular hemolysis as in 1. protozoan diseases e.g. babesiosis, anaplasmosis, trypnosomosis,etc 2. Viral infections like equine infectious anemia 3. Bacterial infections due to Clostridium hemolyticum and leptospirosis 4. Isoimmune hemolytic anemia in newborn foals and piglets. B- Reduced hepatic uptake of bilirubin This has been observed in humnan after administration of certain drugs like rifampin, an antitubercular drug C-impaired conjugation of bilirubin Due to the aquired or hereditary deficiency of gllucuronosyl transferase(GT) Activity of GT is low at birth and normal levels are attained at about 2 weeks. Therefore every newborn develops a mild,transient jaundice –called neonatal or physiological jaundice 2-Toxic or intrahepatic jaundice Injury to hepatiytes and bile canalculi by infectious and non infectious agents eg. Bacteria (salmonella) ---------(infectious canine hepatitis), plant and chemical toxins eg phosphorus, CCl4 Both conjugated and unonjugated bilirubin accumulate in the blood Swelling and disorganization of hepatocytes may compress and block canalculi 3- Obstructive (post hepatic) jaundice There is obstruction to the excretion of conjugated bilirubinin the extrahepatic bile dut system (cholestasis) Obstruction may be caused by 1. Parasites eg cattle and sheep- fasciola hepatica and F. gigantica Pigs- Ascaris lumbricoides Sheep- tapeworms 2. Gallstones 3.Inflammation of bile duct-cholangitis 4.tumours Obstructive (post hepatic) jaundice In case of complete obstruction bile disappears from feces The feces are of grey colour like wet cement but urine is normal in colour Bile is necessary for absorption of fats from intestine Malabsorption of vitamin K predisposes the animals to haemorrhage Accumulation of bile pigments in the skin causes itching(pruritis) Icterus index Test for diagnosis of icterus bycomparison of colour of plasma or serum with a standard solution of potassium dichromate For accuracy, standard should be prepared for each species and breed of animals Van den Bergh reaction Ehrlich’ reagent (diazotized sulphanilic acid) is mixed with plasma or serum The developing coloured compound (azobilirubin) determines the type of bilirubin involved Three interpretations are Direct reaction: pink or purple colour develops immediately and reaches maximum density with 1-2 minutes Indirect or delayed reaction: no colour change in first two minutes. A golden colour develops within 10 minutes.indicates uncojugated bilirubin i.e. hemolytic jaundice Biphasic reaction: a brownish red colour indicates both Comparison of three types of jaundice Ceroid It is acid fast, autoflourescent pigment consisting of partially oxidized and polymerized unsaturated fatty acids. It is an early form of lipofuscin Ceroid is associated with Vit. E deficiency. It does not stain for iron. A. Hepatic ceroidosis Found in salmon and catfish fed rancid diets. Hepatocytes contain acid fast, autoflourescent pigment and liver has brown- orange discoloration. B. Ceroid- lipofuscinosis disease There are a group of diseases in sheep, cattle, goat and dogs characterized by accumulation of fluorescent lipopigments in neuron and other cells Exogenous pigments Most of these are dust particles in the inhaled air, deposited in the lungs and associated lymph nodes. The dust particles act as mild irritants and induce proliferation of fibrous connective tissue( FCT)(fibrosis) and collection of macrophages. The condition is called pneumoconiosis or occupational hazards Particles between 1-5 micrometer diameter are most dangerous Pathogenicity depends upon size, solubility, cytotoxicity and the amount in the inhaled air Phagocytosis of dust particles by alveolar macrophages provides protection 1- Anthracosis It is deposition of carbon or coal dust in the lungs in horses and mules used in coal mines and dogs living in the smoky areas. Tattooing is a localized anthracosis Carbon dust is mildly irritating and causes a slight fibrosis. It is insoluble and persists in the tissues for life 2- Silicosis It is perhaps most prevalent chronic occupational disease associated with silicon industries like glass etc It is a slowly progressive, nodular, fibrosing pneumoconiosis Silica is a powerful irritant and causes extreme fibrosis, predisposing to diseases like tuberculosis 3-Asbestosis The condition is associated with asbestos industries and it is one of the most dangerous pneumoconioses Asbestos particles cause severe irritation and fibrosis Asbestos is carcinogenic 4-Plumbism It is pigmentation in the tissues resulting from the presence of lead and hydrogen sulphide. Lead poisoning may occur from licking of paints or from water in lead pipes Microscopically lead is deposited in the tissues in combination with hydrogen sulphide as a black pigment Photosensitization and photodynamic pigments Photodynamic pigments or agents that make the tissues more sensitive to light. Absorption of certain wavelengths in the sunlight e.g., U.V. light activates these substances and they produce necrosis and edema of these tissues. Continued….. Skin lesions are restricted to hairless and non- pigmented or lightly pigmented areas like teats, udder, ears, eyelids in cows and ears, eyelids, lips and coronets in sheep classification Photosensitization is classified into three types according to the origin and mechanism of photodynamic agent 1. Primary photosensitization Due to the ingestion of pre-formed photodynamic agents in different plants (fagopyrine) and certain drugs like phenothiazine, tetracycline, sulphonamide etc. The agent is deposited in the tissues following absorption in the blood. When such animals are exposed to sunlight, photosensitization occurs. 2-Due to abnormal porphyrin metabolism Congenital porphyria is a hereditary metabolic disorder in cattle and cats with excessive production of two porphyrins (derivatives of hemoglobin): 1-Uroporphyrin and 2-Coproporphyrin Uroporphyrin is deposited in the bones and teeth causing discoloration (pink tooth) 3-Hepatogenous (hepatotoxic) photosensitization This occurs in liver diseases in which injured hepatocytes fail to excrete phylloerythrin. Phylloerythrin is normal end product of chlorophyll metabolism and is photodynamic. This condition is more common in the animals grazing on green pastures