PPT
advertisement

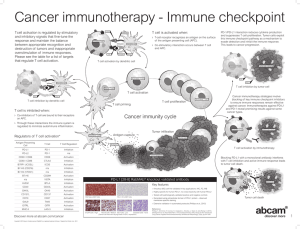
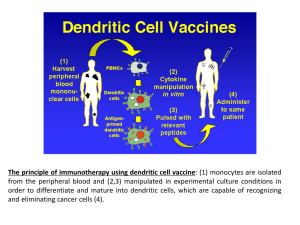
The Promise of Immunotherapy for Cancer Treatment Julie R. Brahmer, M.D., M.Sc. Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins Scott Gettinger 2012 It Takes a Village to Control a T Cell? Tumor Cell or Antigen Presenting Cell T cell Signal 2 B7.1/2 CD28 Signal 1 HLA Class II MHC B7-H1 (PD-L1) Others: ICOS, GITR, Tim-3 antigen CTLA-4 T Cell Receptor LAG-3 PD-1 Blocking the Immune Checkpoint CTLA4 - Ipilimumab Signal 2 B7.1/2 (CD80/86) CD28 CTLA-4 Signal 1 HLA antigen T Cell Receptor T cell Ipilimumab in Melanoma Phase III trial: – 676 patients with chemoresistant metastatic melanoma – Randomized to ipilimumab + gp100, or either treatment alone – Median overall survival of ipilimumab group 10 months, vs 6.4 months for gp100 Immune-related adverse events in 60% of patients (skin, GI, endocrine, liver) FDA decision March, 2011 Hodi et al, NEJM 2010 363:711 Role of PD-1 in Suppressing Antitumor Immunity Activation (cytokines, lysis, prolif., migration) APC T cell B7.1 MHC-Ag Tumor CD28 TCR Signal 1 APC: Antigen presenting cell TCR: T-cell receptor MHC-Ag: Major Histocompatibility Complex-Antigen Keir ME et al, Annu Rev Immunol 2008; Pardoll DM, Nat Rev Cancer 2012 Role of PD-1 in Suppressing Antitumor Immunity Activation (cytokines, lysis, prolif., migration) APC T cell B7.1 MHC-Ag CD28 TCR Signal 1 (-) (-) (-) PD-1 PD-L1 Tumor Inhibition Tumor (anergy, exhaustion, death) Keir ME et al, Annu Rev Immunol 2008; Pardoll DM, Nat Rev Cancer 2012 Role of PD-1 in Suppressing Antitumor Immunity Activation (cytokines, lysis, prolif., migration) APC T cell B7.1 MHC-Ag CD28 TCR Signal 1 (-) (-) (-) AntiPD-1 PD-1 PD-L1 Tumor Inhibition Tumor (anergy, exhaustion, death) Keir ME et al, Annu Rev Immunol 2008; Pardoll DM, Nat Rev Cancer 2012 Clinical Development of Inhibitors of PD-1 Immune Checkpoint Target Antibody Development stage PD-1 NivolumabBMS-936558 Phase III Pembrolizumab MK-3475 Approved 9/4/14 for unresectable or metastatic melanoma MedI-4736 Phase III MPDL-3280A Phase III PD-L1 Durable Responses to Anti-PD-1 OFF THERAPY Pt 22013 0 Pt 14033 CR Stop Rx 1 yr Stop Best Rx resp.(PR) Latest evaluation: CR 2yr 3 yr ? new brain met on MRI resected: - no viable tumor 4 yr 5 yr Latest evaluation: CR Sustained PR 0 1 yr Pt 13019 2 yr 3 yr Stop Best Rx resp.(PR) 4 yr Restart New mets a-PD1 5 yr PD after 2 yr reRx Sustained PR 0 1 yr 2 yr 3 yr 4 yr Lipson E et al Clin Cancer Res 2012 Pembrolizumab in Melanoma Phase Ib trial: 24% response rate in patients with melanoma. Responses lasting for months 1.5-8.4 months. 1 yr survival rate of 58%. 6th drug for melanoma approved since 2011 Ribas A, ASCO 2014, FDA 2014 Broad Activity of PD-1 or PD-L1 Inhibitors Lung Cancer- in Phase 3 trials Kidney Cancer – in Phase 3 trials Bladder Cancer Head and Neck Cancer Ovarian Cancer Multiple cancers are being studied S1400: MASTER LUNG-1: Squamous Lung Cancer- 2nd Line Therapy Biomarker Profiling (NGS/CLIA) CT* Biomarker Non-Match Multiple Phase II- III Arms with “rolling” Opening & Closure PiK3CA Mut PI3Ki CT* Endpoint PFS/OS CCND1, CCND2, CCND3, cdk4 ampl CDK 4/6i CT* Endpoint PFS/OS FGFR ampl, Mut, Fusion FGFRi CT* Endpoint PFS/OS C-MET Expr HGFi+E E* Endpoint PFS/OS CT=chemotherapy (docetaxel or gemcitabine), E=erlotinib PI: V. Papadimitrakopoulou (SWOG) Steering Committee Chair: R. Herbst (YALE, SWOG) Lung Committee Chair: D. Gandara Translational Chair: F. Hirsch Statistical Chair: M. Redman S1400 Master Protocol Unique PrivatePublic Partnerships with the NCTN Alliance SWOG S1400 Master Protocol NCI-C ECOGAcrin NRG Conclusions Immunotherapy agents have promising antitumor activity in multiple cancers Many key questions need to be answered. – How best should these agents be used (by themselves or in combination)? – Who will most likely benefit from these agents (personalize treatment)? Phase 3 trials are ongoing in order to make immunotherapy a reality for the treatment of cancer.