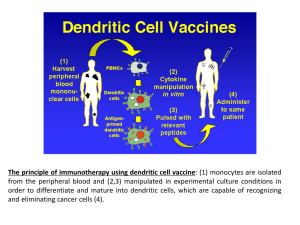
Harnessing the Oncologist That Lies Within A Patient's Immune
advertisement

Immuno-Oncology: Harnessing the Oncologist That Lies Within A Patient’s Immune System Kevin P. Hubbard, DO, FACOI Interim Chair - Department of Specialty Medicine Professor and Chair - Division of Internal Medicine Kansas City University of Medicine and Biosciences - College of Osteopathic Medicine Financial Disclosures I have no real or apparent conflict of interest with the information presented in this lecture Introduction Immuno-Oncology Concepts The immune system Cancer-immunity relationships http://www.medscape.org/viewarticle/844988 http://www.medscape.org/viewarticle/844988 http://www.medscape.org/viewarticle/844988 http://www.medscape.org/viewarticle/844988 Immuno-Oncology Concepts The immune system Cancer-immunity relationships http://www.medscape.org/viewarticle/844988 http://www.medscape.org/viewarticle/844988 http://www.medscape.org/viewarticle/844988 http://www.medscape.org/viewarticle/844988 http://www.medscape.org/viewarticle/844988 http://www.medscape.org/viewarticle/844988 http://www.medscape.org/viewarticle/844988 http://www.medscape.org/viewarticle/844988 New Treatments for Melanoma Historically, a fatal disease once distant metastasis occurs Typically radiation resistant, and chemotherapy regimens minimally effective (15-35% in most series) Immune response modifiers (IL-2, α-interferon) offered modest benefit (10-40%) but with improved progression-free survival compared to cytotoxics New Treatments for Melanoma As a result of several trials, therapies targeting key biochemical pathways have led to new agents with improved responses and more modest toxicities Additionally, agents focused on improving the immune response have demonstrated efficacy in the disease New Treatments for Melanoma Ipilimumab (Yervoy®) Monoclonal antibody directed at the cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) CTLA-4 is involved with immune activity as a “checkpoint” Ipilimumab inhibits CTLA-4, which activates cytotoxic T cells, initiating a cellular response to melanoma (checkpoint inhibition) New Treatments for Melanoma Ipilimumab (Yervoy®) Monoclonal antibody directed at the cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) CTLA-4 is involved with immune activity as a “checkpoint” Ipilimumab inhibits CTLA-4, which activates cytotoxic T cells, initiating a cellular response to melanoma (checkpoint inhibition) New Treatments for Melanoma Ipilimumab (Yervoy®) FDA approved for treatment of metastatic melanoma Improves overall survival Change in “typical” response to treatment…sometimes note increase in tumor size prior to regression of disease (“pseudoprogression”) New Treatments for Melanoma If oncologists can’t use established criteria for response, what will they do? “you gotta know when to hold ‘em, and know when to fold ‘em…” ~Kenny Rogers http://www.medscape.org/viewarticle/830449 New Treatments for Melanoma Ipilimumab (Yervoy®) Toxicities: many are auto inflammatory and due to cytokine release Colitis (8-23%) Hypophysitis (1-5%) Hepatitis (3-7%) Pneumonitis (variable, but almost always <5%) Skin eruptions (0-4%) New Treatments for Melanoma Antibodies targed at PD-1/PD-L1 Nivolumab (PD-1) Pembrolizumab (PD-1) PD-L1 targeted antibodies coming soon! Antibodies Targeted at PD-1 Keynote 002 Trial (Ribas A, et al.; Lancet Oncol 2015; Jun 23. pii: S1470-2045(15)00083-2) 540 patients with ipilimumab-refractory advanced melanoma (and BRAF directed treatment in patients with a BRAF V600 mutation) randomly assigned to pembrolizumab (2 mg/kg every three weeks), pembrolizumab (10 mg/kg every three weeks) or chemotherapy (carboplatin plus paclitaxel, paclitaxel alone, dacarbazine, or temozolomide per institutional standard) Treatment continued until disease progression Antibodies Targeted at PD-1 Keynote 002 Trial (Ribas A, et al.; Lancet Oncol 2015; Jun 23. pii: S1470-2045(15)00083-2) Chemotherapy (control group) Pembrolizumab 2mg/kg Pembrolizumab 10 mg/kg Progression-free survival (hazard ratio) - 0.57 (95% .45-.73; p<.0001) .50 (95% CI .39-.64; p<.0001) Progression-free survival (6-month) 16% 34% 38% Grade 3-4 Toxicity 26% 11% 14% Antibodies Targeted at PD-1 Nivolumab Previously untreated patients → Checkmate 066 Trial (Robert C; et al.; N Engl J Med. 2015;372(4):320) 418 previously untreated patients with wild type BRAF melanomas randomly assigned to nivolumab (3 mg/kg every two weeks) or dacarbazine (1000 mg/m2 every three weeks) Antibodies Targeted at PD-1 Checkmate 066 Trial (Robert C; et al.; N Engl J Med. 2015;372(4):320) Dacarbazine Nivolumab Response Rate 14% 40% Duration of Response (months) 2.2 5.1 12-month Response Rate 42% 73% (HR) for death 0.42, 99.8% CI 0.25-0.73 Antibodies Targeted at PD-1 Nivolumab Previously treated patients → Checkpoint 037 trial (Weber JS, et. al.; Lancet Oncol. 2015;16(4):375) 405 patients with prior anti-CTLA4 therapy (and BRAF directed treatment in patients with a BRAF V600 mutation) randomly assigned in a 2:1 ratio to either nivolumab or chemotherapy (either dacarbazine or carboplatin plus paclitaxel) Results of planned interim analysis are based upon 167 patients (120 treated with nivolumab and 47 treated with chemotherapy) with a minimum follow-up of ≥6 months Antibodies Targeted at PD-1 Checkpoint 037 trial (Weber JS, et. al.; Lancet Oncol. 2015;16(4):375) Chemotherapy Nivolumab Response Rate 10% 32% Median Duration of Response (months) 3.5 Not yet reached at time of analysis Tumor responses were seen with nivolumab in patients with BRAF mutations who had progressed on a prior BRAF inhibitor (6/26 = 23%) and appeared to be independent of benefit from prior ipilimumab treatment Current Management of Melanoma The role of PD-1 directed therapies has revolutionized treatment of this disease Emerging data sets suggest improved responses to multiagent therapies (PD-1 plus CTLA-4 or PD-1 plus BRAF or MEK inhibition) Newer Approaches in NSCLC Patients with Stage I, II, or III NSCLC are generally treated with curative intent using surgery, chemotherapy, radiation therapy (RT), or a combined modality approach Factors affecting choice of treatment Extent of disease Characterization of the tumor (histology, driver mutation status) Patient-specific issues (age, co-morbidities, performance status) Newer Approaches in NSCLC Systemic therapy options Cytotoxic chemotherapy Targeted agents Monoclonal antibodies Choices depend on molecular and histologic features of the tumor, presence of a driver mutation Age of patient factors in as well (older patients are less well studied) Newer Approaches in NSCLC “Driver mutation” — occur in cancer cells with mutations in genes encoding for proteins critical to cell growth and survival Typically not found in the germ line (normal) cells, thus allows for targeting of malignant cells Immunotherapy in Lung Cancer??? For many years, bronchogenic carcinoma not considered immunogenic Phase I study of nivolumab in previously treated NSCLC (Raez LE, et al. Clin.Med. Res. 2005. 3:221-228) ) Nivolumab - fully human PD-1 monoclonal antibody Overall response 18%, OS 9.9 months, 1-year survival 42% Immunotherapy in Lung Cancer??? Phase 2 study of nivolumab in refractory squamous cell NSCLC demonstrated 14.5% overall response rate (Topalian SL, Hodi FS, Brahmer JR. New Engl Jour Med 2012; 366:2443) Durable responses noted, lasting several months Most patients treated with conventional therapy who respond have a response duration of a few weeks Immunotherapy in Lung Cancer??? http://www.medscape.org/viewarticle/844988 Improved response and overall survival vs docetaxel Immunotherapy in Lung Cancer??? As a result of this data, the US Food and Drug Administration granted approval on March 4, 2015 of nivolumab for the treatment of patients with advanced squamous NSCLC with progression on or after platinum-based chemotherapy Immunotherapy in Lung Cancer??? Pembrolizumab→KEYNOTE 001 Trial (Geron EB, et al.; N Engl J Med 2015; 372:20182028) Phase I trial of 495 patients receiving pembrolizumab at a dose of either 2 mg/kg or 10 mg/kg every 3 weeks or 10 mg/kg every 2 weeks Assigned to either a training group (182 patients) or a validation group (313 patients) PD-L1 expression in tumor samples assessed using immunohistochemical analysis, with results reported as the percentage of neoplastic cells with staining for membranous PD-L1 (proportion score) Immunotherapy in Lung Cancer??? KEYNOTE 001-Results (all patients on treatment) (Geron EB, et al.; N Engl J Med 2015; 372:2018-2028) Objective response rate 19.4% Median duration of response 12.5 months Median duration of progression-free survival 3.7 months Median duration of overall survival 12 months PD-L1 expression in at least 50% of tumor cells correlated with improved efficacy→ORR in that group was 45.2% median overall survival not reached at time of data analysis Immunotherapy in Lung Cancer??? Toxicities to PD-1/PD-L1 therapies General: fatigue, rash, pruritis, myalgias, loss of appetite On occasion: colitis, thyroiditis, elevation of liver transaminases, autoimmune disorders Points to Remember… Most cancers stimulate the immune system Therapeutic immunity can be either passive or active Passive = supplying an antibody response Active = vaccination to achieve an antibody response Therapeutic approaches now available can stimulate immunity and address mechanisms of immune What’s Coming Down The Pike? Beyond melanoma and NSCLC, CTLA-4-and PD-1-directed therapies are being evaluated for the treatment of prostate cancer, renal cell carcinoma, breast cancer, bladder cancer, colorectal cancer, gastric cancer, and hematologic malignancies The use of CTLA-4 and PD-1-directed therapies are being explored in combination with conventional chemotherapy in a wide variety of malignancies, including NSCLC, melanoma, breast cancer, and prostate cancer Conclusions There is a close relationship between the immune system and cancer control, growth, and metastasis Novel therapies targeting these relationships are being developed for a wide variety of malignancies, and hold promise for effective treatment and manageable side effects The role of PD-1/PD-L1 and CTLA-4 directed therapies in combination with other modalities is still being explored things are changing quickly… Stay Tuned!!! Questions?