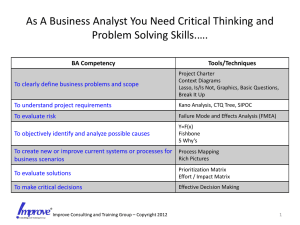
Risk Management Tools, Techniques and Tribulations
advertisement

Risk Management Tools, Techniques and Tribulations: The Pain and Recovery in Life Sciences Project Management Institute – SF Bay Area Chapter Embassy Suites, Walnut Creek March 17, 2010 Moderator Tim Salaver, MBA, PMP, CSSMBB Principal, Cornerstone Systems Solutions President, Golden Gate Chapter of APICS The Association for Operations Management EVP, Operations and Technology, Bio Supply Management Alliance Director, Certification Preparation, PMI-SFBAC 2 Life Science Risk Management 3/17/2010 Supply Chain Risk Management Bill Coakley, MBA Sr. Director, Supply Chain Management, SciClone Pharmaceuticals Chairman, Sourcing Management Steering Committee, Bio Supply Management Alliance 3 Life Science Risk Management 3/17/2010 Project Risk Management Linda Karr, MBA, PMP Project Manager, Pharma Technical Development, Genentech President, San Francisco Chapter of International Society for Pharmaceutical Engineering (ISPE) 4 Life Science Risk Management 3/17/2010 Enterprise & Operations Risk Management Chris Sam, MS, Risk Management Executive Director, Craigshannock Member, Risk Management Steering Committee, Bio Supply Management Alliance Executive Committee member of the Strategic Risk Management Council of the Conference Board Former Executive Director, Operations Risk Management, Amgen 25 years in energy industry, previously at ExxonMobil 5 Life Science Risk Management 3/17/2010 Industry Risk Management Jane Lavine, MBA, CPCU, ARM, CFE Life Science Risk Management consultant Former VP, Life Science Practice, Marsh 6 Life Science Risk Management 3/17/2010 The Healthcare System 7 Life Science Risk Management 3/17/2010 The Healthcare Shuffle Rx Delivery System Biotechnology/ Pharmaceutical manufacturer Patient’s Pharmacy Specialists Rx Gov. Health Agencies Referring MD Compliance Orders Lab Tests X-rays Pathology Etc. Orders Rx Diagnosis Diagnosis Patient’s Medical Record Provider Patient’s Insurance Provider Database Coverage, Coding & Approvals Interactions & Alternatives Interactive Prescription Database Patient’s Primary Care MD Pocket PC 8 Life Science Risk Management 3/17/2010 The Life Sciences Biotechnology Pharmaceuticals Medical Devices Diagnostics Nutraceuticals Animal Biologics Distributors or Delivery System (e.g., Cardinal Health, McKesson, Amerisource-Bergen) Healthcare Providers 9 Life Science Risk Management 3/17/2010 Managing Risks Related to Pharmaceuticals and Biotechnology Tremendous Opportunity for Improvement in Healthcare Electronic Medical Records Standardization Initiative The Technology exists to Revolutionize Healthcare (not just reform) Driven By Department of Health – Bypassing Standards Bodies Calling for Compliance by 2015 Save Lives Reduce Costs Improve Quality of Care The Strategy and Relationships are mostly in Place Risks remain high for the companies manufacturing the drugs….WHY? 10 Life Science Risk Management 3/17/2010 Audience question: What happens prior to the drugs getting to the pharmacy? 11 Life Science Risk Management 3/17/2010 Project Risk Management in the Drug Development Pipeline 12 Life Science Risk Management 3/17/2010 Typical Drug Development Pipeline Product/Process Development Panel Question At what point in the pipeline do you get involved and what is your role in managing risk? Product/Process Development 14 Life Science Risk Management 3/17/2010 Panel Question In your role, do you have the ability to pull the “andon” cord? 15 Life Science Risk Management 3/17/2010 Panel Question In your specific role, how important are you in managing risks to the patient? This question is important to note because while the manufacturer does not have a direct relationship to the patient, the quality of care is dependent on the quality of the product. 16 Life Science Risk Management 3/17/2010 Panel Question What tools and techniques do you employ to identify risks? 17 Life Science Risk Management 3/17/2010 Project Risk Management in the Product Life Cycle 18 Life Science Risk Management 3/17/2010 PMI Risk Management Process Pre-Launch: Commercialization Product Life Cycle What inherent risks are addressed prior to product launch? Product/Process Development 20 Life Science Risk Management 3/17/2010 Post-Launch: Commercialization Product Life Cycle What steps do you take to mitigate risks post commercialization? Product/Process Development 21 Life Science Risk Management 3/17/2010 Risk Management Tools and Techniques 22 Life Science Risk Management 3/17/2010 Risk Register - example What is this 1 risk? 2 3 23 0 0 0 Category ID Risk Description Probability Impact Detectability Importance Project Project manager Project artifacts Risk Management Matrix (Risk Register) Project title here Project # Project manager name here Sponsor Location of project documents here Updated Trigger Event/Indicator What act or event initiates either the risk occurrence or precipitates the response strategy? Risk Response and Description How will you respond to this risk and what actions will you take to match that response? Contingency Plan If the risk becomes a reality, what will you do in response, as a backup, or alternative/ Life Science Risk Management Project # here Sponsor name here Date of update here Owner Status Who monitor s this risk? 3/17/2010 Date Entered Date to Review Risk and Response Log - example Project Identification Project Name Project Manager Project Team(s) Project Stage Project Team Review Date <Name of project> <Name of project manager> <Name of team or teams using log> <Name of current stage> <Name of assigned Project Team Leader> <Date of last review with team> Purpose: To document, track and review risks throughout the life of the project. A "risk" is an uncertain event or condition, which if it occurs, may have a positive or negative affect on a project. Risk and Response Log ID # Prob <###> <High, Med, Low> Impact Team Risk Event <High, <Identify the <Briefly describe the risk event> Med, team/sub-team Low> that is responsible for monitoring/man aging identified risk.> Risk Impact Description <Describe the impact of the risk event should it occur. Provide both qualitative information and a quantitative estimate> Strategy <Avoid/Mitigat e, Active Acceptance, Passive Acceptance> Risk Response Plans <Describe what will be done in an attempt to avoid the risk event or what will be done to mitigate the risk event should it occur. Include any commentary as needed.> 002 003 004 24 Life Science Risk Management 3/17/2010 Risk Owner <Identify by name and role the individual that owns the risk event> Risk Map (source: Marsh) Map Key Clinical trials 2. Sales and marketing practices 3. Consumer satisfaction/Company reputation 4. Environmental compliance 5. Business Interruption 6. Employee health and safety 7. Contingent BI- sole source supplier 8. Intellectual Property Infringement 9. Transit/supply chain 10. International regulations 1. How to use the Risk Map The dots represent a sample of risks associated with a life science company Each company risk profile is unique The positioning of the risk dots is both qualitative and quantitative analysis The visual format is used to review the different risk profiles and their relationship to each other This tool can be used for a function or a specific process Failure Mode and Effects Analysis - example Title:Risk Management Presentation FMEA #:1 Product Name:Professional Development Meeting Originator:Tim Salaver Process:Awareness and development of RM tools Issue Date:3/17/2010 Equipment: Revision Date: Participants:PMI Location:Embassy Suites, Walnut Creek Type of FMEA:P-FMEA FMEA Types:System Design, Detailed Design, Process, Equipment Control(s) Containment 3 Presenter Experience 2 None 10 12 Review of training Tim Salaver 60 results and prepare on 03/17/10 and Panelists weak items 0 Mostly Developed Ahead of Presentation 3 None 10 60 200 Have Time to have Tim Salaver Materials Proofed 03/17/10 and Panelists Independently 0 Experience 2 None 10 72 360 Adjust materials to be Tim Salaver covered or lengthen 03/17/10 and Panelists time 0 0 0 in place Date Risk Management Presentation Instructor Lack of Learning Transfer 2 Risk Management Presentation Materials Lack of Understanding 4 Risk Management Presentation Time Lack of Time To Fully Explain Content The presentation is 9 not comprehensive 4 enough 27 Not Enough Time 5 To Prepare Actions Taken Life Science Risk Management RPN 3 in place Action(s) of Failure Incompetent Presenters Action Results Due Detection 3 Mechanism(s) Owner Occurrence 2 failure Recommended Severity 2 mode People Cause(s)/ Current RPN 2* Effect(s) of Current RPN 1* Failure Potential Detection 2 Machine Potential Detection 1 Process Potential Occurrence 1 Failure Item: Severity 1 * RPN = Risk Priority Numbe r 0 3/17/2010 Workshop on Preparing an FMEA Failure Mode and Effects Analysis is an effective tool in managing risks 28 Life Science Risk Management 3/17/2010 FMEA Process Analysis of potential failure modes within a system for classification by severity or determination of the effect of failures on the system Widely used in manufacturing industries in various phases of the product life cycle Increasingly finding use in the service industry Failure modes are any errors or defects in a process, design, or item, especially those that affect the customer, and can be potential or actual Effects analysis refers to studying the consequences of those failures Example FMEA Worksheet Function Failure mode 29 Effects S (severity rating) O Cause(s) (occurrence rating) Current controls D (detectio n rating) CRIT (critical characte ristic Life Science Risk Management RPN (risk priority number) Responsi Recomm bility and ended target actions completi on date 3/17/2010 Action taken FMEA Terms Failure mode - A failure mode is an event that causes a loss of a required function. Failure effect - Immediate consequences of a failure on operation, function or functionality, or status of some item. Indenture levels - An identifier for item complexity. Complexity increases as levels are closer to one. Local effect - The Failure effect as it applies to the item under analysis. Next higher level effect - The Failure effect as it applies at the next higher indenture level. End effect - The failure effect at the highest indenture level or total system. Failure cause - Defects in design, process, quality, or part application, which are the underlying cause of the failure or which initiate a process which leads to failure. Severity: - The consequences of a failure mode. Severity considers the worst potential consequence of a failure, determined by the degree of injury, property damage, or system damage that could ultimately occur. Example FMEA Worksheet Function Failure mode 30 Effects S (severity rating) O Cause(s) (occurrence rating) Current controls D (detectio n rating) CRIT (critical characte ristic Life Science Risk Management RPN (risk priority number) Responsi Recomm bility and ended target actions completi on date 3/17/2010 Action taken 31 Life Science Risk Management 3/17/2010 Step 1 – Detect a Failure Mode A worksheet needs to be created, which contains the important information about the system, such as the revision date or the names of the components. On this worksheet all the items or functions of the subject should be listed in a logical manner, based on the block diagram. Describe the system and its function. Good understanding simplifies further analysis. Consider both intentional and unintentional uses. A block diagram of the system needs to be created. Example FMEA Worksheet Function Failure mode 32 Effects S (severity rating) O Cause(s) (occurrence rating) Current controls D (detectio n rating) CRIT (critical characte ristic Life Science Risk Management RPN (risk priority number) Responsi Recomm bility and ended target actions completi on date 3/17/2010 Action taken FMEA Cause and Effect Diagram 33 Life Science Risk Management 3/17/2010 Step 2 - Severity Determine all failure modes based on the functional requirements and their effects A failure mode in one component can lead to a failure mode in another component Write these effects down in terms of what the user might see or experience Examples: degraded performance, noise or even injury to a user Each effect is given a severity number (S) from 1 (no danger) to 10 (critical) Prioritize the failure modes and their effects Severity 9 or 10 actions are considered changes in design by eliminating the failure mode Example FMEA Worksheet Function Failure mode 34 Effects S (severity rating) O Cause(s) (occurrence rating) Current controls D (detectio n rating) CRIT (critical characte ristic Life Science Risk Management RPN (risk priority number) Responsi Recomm bility and ended target actions completi on date 3/17/2010 Action taken Step 3 - Occurrence Detailed development section of the FMEA process Necessary to look at the cause of a failure and how many times it occurs A failure cause is looked upon as a design weakness All the potential causes for a failure mode should be identified and documented Examples of causes are: erroneous algorithms, excessive voltage or improper operating conditions A failure mode is given an occurrence ranking (O), again 1–10 Example FMEA Worksheet Function Failure mode 35 Effects S (severity rating) O Cause(s) (occurrence rating) Current controls D (detectio n rating) CRIT (critical characte ristic Life Science Risk Management RPN (risk priority number) Responsi Recomm bility and ended target actions completi on date 3/17/2010 Action taken Step 4 - Detection When appropriate actions are determined, test their efficiency The proper inspection methods need to be chosen How likely a failure can be identified or detected. Each combination from the previous 2 steps receives a detection number (D). Assigned detection number measures the risk that the failure will escape detection. A high detection number indicates that the chances are high that the failure will escape detection, or in other words, that the chances of detection are low. Example FMEA Worksheet Function Failure mode 36 Effects S (severity rating) O Cause(s) (occurrence rating) Current controls D (detectio n rating) CRIT (critical characte ristic Life Science Risk Management RPN (risk priority number) Responsi Recomm bility and ended target actions completi on date 3/17/2010 Action taken Risk Priority Numbers RPN = S × O × D Easy to determine the areas of greatest concern Modes that have the highest RPN should be given the highest priority for corrective action Whenever a design or a process changes, an FMEA should be updated Example FMEA Worksheet Function Failure mode 37 Effects S (severity rating) O Cause(s) (occurrence rating) Current controls D (detectio n rating) CRIT (critical characte ristic Life Science Risk Management RPN (risk priority number) Responsi Recomm bility and ended target actions completi on date 3/17/2010 Action taken Exercise Can you determine the order of need for change in the following three examples: Severity (5), Occurrence (4), Detection (2) = 40 Severity (9), Occurrence (2), Detection (2) = 36 Severity (8), Occurrence (1), Detection (8) = 64 The correct order for action is #2, #1, #3. Why? Example FMEA Worksheet Function Failure mode 38 Effects S (severity rating) O Cause(s) (occurrence rating) Current controls D (detectio n rating) CRIT (critical characte ristic Life Science Risk Management RPN (risk priority number) Responsi Recomm bility and ended target actions completi on date 3/17/2010 Action taken Next Steps Eliminate the failure mode with severity of 9 or 10 Minimize the severity of other failure modes Reduce the occurrence of the failure mode Improve the detection (reduce the number) Update the FMEA 39 At the beginning of a cycle Changes are made to the operating conditions A Change is made in the design New regulations are instituted Customer feedback indicates a problem Life Science Risk Management 3/17/2010 Advantages Improve the quality, reliability and safety of a product/process Improve company image and competitiveness Increase user satisfaction Reduce system development timing and cost Collect information to reduce future failures, capture engineering knowledge Reduce the potential for warranty concerns 40 Early identification and elimination of potential failure modes Emphasize problem prevention Minimize late changes and associated cost Catalyst for teamwork and idea exchange between functions Reduce the possibility of same kind of failure in future Life Science Risk Management 3/17/2010 Sample BioPharma Case presented by Chris Sam Intro to Cp and Cpk (Measurements of process capabilities used in quality analysis.) Cp - Inherent Process Capability Note: in some industries this calculation is called pk. This is the ratio of the Upper Specification Limit minus the Lower Specification Limit to six sigma. It is denoted by the symbol Cp. Cp = (Upper Spec Limit - Lower Spec Limit) 6Sigma Actual Cannot calculate Cp if specifications are one sided. In other words, if specification only has an upper parameter specification limit or a lower specification limit, Cp is ignored and only Cpk can be used. Confusion about the difference between Cp and Cpk. The difference is in calculating the actual sigma or standard deviation, either using an estimate, or the actual calculations. Terms are sometimes used interchangeable, even though they are not. the same. Statistics IS a science, and as such, all theory should be able to be validated by such peer reviews. 41 Life Science Risk Management 3/17/2010 Sample BioPharma Case presented by Chris Sam Introduction to FMEA software You help to complete the missing information Using Cp and Cpk analysis 42 Life Science Risk Management 3/17/2010 Q&A 43 Life Science Risk Management 3/17/2010 Additional Resources APICS The Association for Operations Management (www.apics-ggc.org) Bio Supply Management Alliance (www.biosupplyalliance.org) International Society for Pharmaceutical Engineers (www.ispe.org) Strategic Risk Management Council (www.conferenceboard.org) Marsh Risk Consulting (www.marshriskconsulting.com) For more information, please send email to tim@biosupplyalliance.org 44 Life Science Risk Management 3/17/2010 THANK YOU FOR YOUR PARTICIPATION Have a safe and risk-free drive home!!!