Finish 1s electron lab, orbital diagrams, Hund`s rule, Pauli exclusion
advertisement

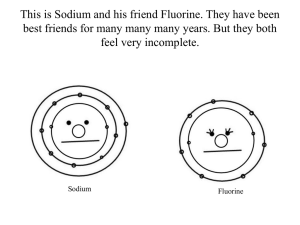
Finish 1s electron lab, orbital diagrams, Hund’s rule, Pauli exclusion, valance electrons • Objective – Today I will be able to: • Construct a model of where electrons in the 1s orbital are located. • Explain the behavior of electrons based on Pauli’s exclusion Principle and Hund’s Rule • Illustrate the location of an electron by drawing an orbital diagram • Identify the location of valance electrons in an atom • Evaluation/ Assessment – Informal assessment – student responses when reviewing electron configuration problems, orbital diagrams and valence electrons. Listening to group interactions on practice sheets and lab – Formal Assessment – collecting and analyzing responses to electron configuration worksheet 2 and the where is the electron lab. Reviewing responses to the exit ticket Lesson Sequence • Warm – Up • Explore: Students will be sorted into groups and will complete the where is the 1s electron lab located – I will be assessing students progress during the lab as I monitor group discussions • Evaluate: Students will complete an electron configuration WS (assessment) • Explain: principles, rules and diagrams of electron configurations • Elaborate: Students complete orbital diagrams worksheet • Evaluate: Students will draw orbital diagrams on the board and explain their answers (informal assessment) • Explain: Valance Electrons Notes • Elaborate: Students complete a valance electrons worksheet • Evaluate: Students will share responses to the worksheet • Exit Ticket Warm - Up Write the electron configuration for the following elements: • Magnesium • Iron • Arsenic What is the abbreviated configuration for Sulfur? Objectives • Today I will be able to: – Construct a model of where electrons in the 1s orbital are located. – Explain the behavior of electrons based on Pauli’s exclusion Principle and Hund’s Rule – Illustrate the location of an electron by drawing an orbital diagram – Identify the location of valance electrons in an atom Homework • Finish Orbital Diagrams WS Agenda • • • • • • • • • Warm – Up Finish Lab (15 minutes) Electron Configuration Practice Notes Pauli Exclusion Principle, Hund’s Rule, Orbital Diagrams Orbital Diagram Practice Review Practice as a class Valence Electron Notes Valance Electron Practice Exit Ticket Finish the where is the electron lab? When you finish the lab, hand in one copy per group. Staple together your lab sheet, the answers to the questions, your graph and your target. Turn it in on my desk and take a copy of the electron configurations practice worksheet and begin to work on it. Complete the Electron Configuration Practice Worksheet Hand in upon completion Electron Configuration Rules and Principles Pauli Exclusion Principle • • • • An orbital can hold a max of 2 eTo occupy the same orbital, they must spin in opposite directions If 2 e- occupy an orbital, they are said to be “paired” If only 1 e- is present in an orbital, it is “unpaired” Pauli Exclusion Principle Hund’s Rule • e- occupy orbitals so that a max number of unpaired e- result • More stable arrangement Orbital Diagram • Shows the electrons in their sublevels • Represented with arrows Orbital Diagram 1s 2s 2px 2py 2pz H ↑ He ↑↓ C ↑↓ ↑↓ ↑ ↑ Ne ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ Complete the Electron Configuration and Orbital Diagram Practice Worksheet Review the Electron Configuration and Orbital Diagram Practice Worksheet Valence Electron Notes Valence Electrons • Electrons in the outermost energy level • Determines the number of electrons an atom gains, loses, or shares • These are the electrons that are involved in bonding Valence Electrons (skip) • Write the electron configuration for oxygen • 1s2 2s2 2p4 • Oxygen has six valence electrons (2s2 and 2p4) Valence Electrons (skip) • Write the electron configuration for potassium • 1s2 2s2 2p6 3s2 3p6 4s1 • Potassium has 1 valence electron (4s1) Valence Electrons • • • • Exceptions are the d and f sublevels! Use the number of electrons in the last s sublevel (and the p sublevel, if available) Write the electron configuration for Bromine 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5 Bromine has seven valence electron (4s2 and 4p5) Foreshadowing: Octet Rule • Most atoms want 8 electrons in their outermost level OR full s and/or p sublevels • Atoms will form bonds to achieve the desired amount of electrons • Atoms are most stable when they have a full outer shell Complete Valence Electrons Worksheet Review Valence Electrons Worksheet as a class Exit Ticket • For the element Chlorine: – Write the electron configuration – Write the abbreviated electron configuration – Draw an orbital diagram • Explain why you drew the electrons in the location of the orbital's that you did – Determine the number of valence electrons