File
advertisement

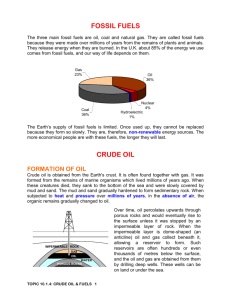
L01. Making Crude Oil Useful Lesson Outcomes APP: AF1,AF3,AF4 HSW: 2b,2c,3a Task 1: Explain what fossil fuels are. Grade C Task 2: Describe: • How crude oil is separated into fractions • The main fractions obtained from crude oil Grade B Task 3: Explain why crude oil can be separated into fractions Grade A/A* How I did Targets Connector: (Grade D) Describe, in as much detail as possible, how fossil fuels were made. Formation of oil and gas 1) Layers of dead sea _____ settle on the seabed. 2) Layers of __________ rock build up on top. 3) The heat and ________ from these rocks, along with the absence of ______, mean that oil and gas are formed over ______ of years. Words – sedimentary, millions, oxygen, creatures, pressure Homework • Homework task: 1. Produce a poster to show Fractional distillation and the uses of the fractions produced. • Due date: • Criteria for Grade C: Describes the main fractions obtained from crude oil • Criteria for Grade B: Explains how crude oil is separated into fractions. • Criteria for Grade A/A*: Explains why crude oil can be separated into fractions BIG picture • What skills will you be developing this lesson? • • • • • • • • • ICT Numeracy Literacy Team work Self management Creative thinking Independent enquiry Participation Reflection • How is this lesson relevant to every day life? (WRL/CIT) Task 1 (Grade C) Task 1: Explain what fossil fuels are. Keywords for Task 1: •Fossil fuels •Natural gas •LPG •Methane •Propane •Butane •Petrol Task 1: Extension •Paraffin •Diesel •Coal New Information for Task 1 • Watch presentation_001. • Now write down one thing you know about fossil fuels • Share your answer with a partner. • Pairs feed back one of their answers to the rest of the class, ‘passing’ if their answer has already been given. Fossil fuels were formed from the remains of dead plants and animals, that lived million of years ago. Burning fossil fuels - gases Natural gas (methane) is a fossil fuel. LPG Propane and butane are the liquefied petroleum gases (LPG) most commonly used. Propane has a variety of uses, including heating homes, and as a fuel for vehicles. Butane is sold as camping gas. Demonstration – Burning fossil fuels Petrol, paraffin and diesel are examples of liquid fossil fuels. Coal is a solid fossil fuel. Task 1: Answers • What are fossil fuels? Fossil fuels were formed from the remains of dead plants and animals, that lived million of years ago. • Name the fossil fuels mentioned so far (7). Natural Gas (Methane), LPG (Propane), Camping Gas (Butane), Petrol, Paraffin, Diesel and Coal Task 1: Review Go back to your lesson outcome grid and fill out the ‘How I did’ and the ‘Targets’ column. Lesson Outcomes Task 1: Explain what fossil fuels are. Grade C How I did Met? Partly met? Not met? Targets How can I improve on task 1? Task 2 (Grade B) • Task 2: • Describe: • How crude oil is separated into fractions • The main fractions obtained from crude oil • Task 2: Extension Keywords for Task 2: • hydrocarbons • non-renewable resource • finite resource New Information for Task 2 Crude oil is: • the source of liquid fossil fuels. • a mixture of hydrocarbons. • a non-renewable resource. • a finite resource. Explain what the words in WHITE mean. Hydrocarbons Hydrocarbons are compounds made from hydrogen and carbon ONLY. Crude oil is a non-renewable resource, because … .. it has taken millions of years to form from the remains of plants and animals. Renewable resources must be … .. replaceable within a reasonable amount of time (days to years). Crude oil is a finite resource, because … .. there is only so much of it on planet earth, and eventually it will be all used up. Fractional distillation of crude oil Small molecules: LPG • Low boiling points • Very volatile Petrol • Flows easily • Ignites easily Crude oil is a mixture of HYDROCARBONS and these can be separated due to differences in their BOILING POINTS. Large molecules: • high boiling points http://www.blackgold.a b.ca/ict/Division4/Scien ce/Div.%204/distillation /Fractional/main.swf • not very volatile • viscous • does not ignite easily Task 2: Summary 1. Copy and complete: Crude oil is a mixture of many different hydrocarbons. Crude oil is separated by fractional distillation. Fractions containing mixtures of hydrocarbons are obtained. Each fraction contains many substances with similar boiling points. 2. The fractions collected from the cooler top to the hotter bottom of the fractional distillation column are: LPG, petrol, paraffin, diesel, heating oil, fuel oil, bitumen Use their first letters to make a rhyme to help you remember them. Task 2: Review Go back to your lesson outcome grid and fill out the ‘How I did’ and the ‘Targets’ column. Lesson Outcomes Task 2: •How crude oil is separated into fractions •The main fractions obtained from crude oil Grade B How I did Met? Partly met? Not met? Targets How can I improve on task 2? Task 3: (Grade A/A*) • Task 3: Explain why crude oil can be separated into fractions. Keywords for Task 3: • forces See page 5 in your text books, and using diagrams, and the key words, write your own explanation. • intermolecular • Task 3: Extension graph work slide • chains • molecules • attraction • energy Task 3: Review Go back to your lesson outcome grid and fill out the ‘How I did’ and the ‘Targets’ column. Lesson Outcomes Task 3: Grade A/A* How I did Met? Partly met? Not met? Targets How can I improve on task 3? Review of lesson - Keywords: • • • • • • Crude oil, finite resource, fossil fuel, fraction, fractional distillation, non-renewable resource. Put your hand up and explain the meaning of one of these. Investigating crude oil Hydrocarbon formula Boiling point Relative oC mass CH4 15 16 C8H18 40 114 C15H32 180 212 C22H46 310 C30H62 340 422 C50H102 420 702 Plot an xy-graph of relative mass v boiling point Draw a line/curve of best fit and use the graph to predict the missing boiling point Technicians’ notes Demo 1 – burning fossil fuels Eye protection Safety screen Three teat pipettes Ceramic tile or tin lid Heatproof mat Diesel Paraffin Petrol Coal Demo 2 – Fractional distillation in the lab Ask technicians to set up this apparatus: