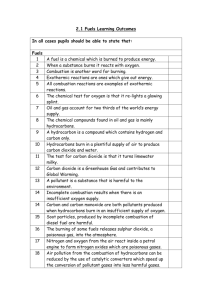
Fossil fuels: coal, oil and natural gas, were formed millions of years
advertisement

Fossil Fuels - Summary Fossil fuels: coal, oil and natural gas, were formed millions of years ago from decaying plant and animal matter trapped under sand and mud. High pressure and temperature transformed the plant material into coal and the animal matter into oil. The supply of fossil fuels is finite, and the rate at which they are being used will eventually lead to a fuel crisis - there will not be enough fuel for transport or industry. Coal and oil are also used to generate electricity, and as starting materials for the production of medicines, plastics, dye stuffs, paints and fertilisers. A shortage will have a dramatic effect on our lifestyles. Crude oil is a mixture of compounds, mainly compounds of carbon and hydrogen only called hydrocarbons. Since different substances have different melting points and boiling points, the components of crude oil can be separated by fractional distillation. The crude oil is heated until the boiling point of one of the compounds is reached – this evaporates and the gas bubbles up through the mixture. The gas is then separated from the mixture and when cooled it condenses and can be collected outside the container. The temperature of the mixture is raised again to the boiling point of the next component. In practice, the boiling points are too close to allow complete separation, and three or four components will evaporate within a small temperature range - this is called a fraction. The first fractions boil at low temperatures and evaporate readily (highly volatile). They are thin liquids with high flammability - they burn well - and are mainly used in combustion engines. The later fractions become increasingly more viscous (thicker) and evaporate very slowly. They do not burn well and are used as lubricating oils, tars and waxes. The first fractions are the short chain small molecules. Later fractions are longer chain, larger molecules that evaporate and burn with difficulty. The larger molecules have higher boiling points, as more energy is required to change the molecules from the liquid to the gaseous state. Different crude oils from different parts of the world contain varying amounts of each fraction. Hydrocarbons burn to give out energy in exothermic reactions. The combustion, or burning in oxygen, of hydrocarbons produces carbon dioxide and water. Methane burns in air (80% nitrogen, 20% oxygen) and the products of combustion are drawn through the apparatus by a pump. Condensation forms in the U tube - An accurate test for water is that its boiling point is 100×C, but there is not enough formed for testing - and the lime water turns milky, showing that carbon dioxide is also a combustion product. If carbon dioxide and water are produced from burning a fuel, it must have contained Carbon and Hydrogen. The oxygen comes from the air. The Greenhouse Effect: Burning fossil fuels creates large amounts of carbon dioxide in to atmosphere. This is thought to form a layer around the earth in the upper atmosphere that allows heat in from the sun, but does not allow the earth to lose heat by radiation. This results in an increase in global temperatures, changes in climate patterns and, as the polar ice cap melts, rising sea levels. Oil Spillage: The transportation of large amounts of crude oil around the world in tankers inevitably results in oil spillage at sea. This causes major damage to coastline plant and animal life.