eIRB 2 Information Session
advertisement

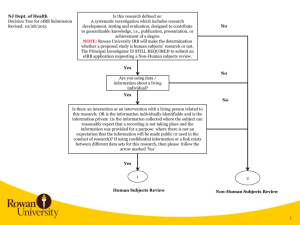
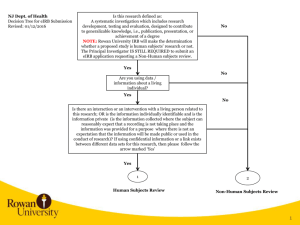
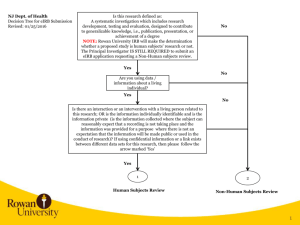
eIRB 2 Rational 2004 – Launch of eIRB 2009 – Last major upgrade 2011 – Last quarterly update eIRB Today: Outdated architecture Increasing memory issues Slow processing speed Reached capacity of data storage: 5000+ approved/active applications 18,000+ total protocols (new and terminated) 23,000+ PIs and study team members eIRB 2 Rational 2012 – The Office of Human Subjects Research (OHSR) and the Office of Research Environment System (RES) began the task of rebuilding the system, with the newest architecture on the latest software platform available. eIRB User Impact Q: Where will I find my studies when eIRB2 goes live? eIRB 1 All Further Study Actions (FSAs) approved prior to the update – they will not transfer to eIRB2. Disapproved, Expired, Terminated and Withdrawn Applications Applications with a pending action The current application will automatically transfer to eIRB2 when the pending action(s) are Approved. No new submissions can occur in eIRB1 after the eIRB2 go live date. Investigators will have 90 days (from the eIRB2 launch date) to submit ANY FSA in “Researcher Prep”. eIRB 2 New Applications in “Researcher Prep” < 90 days Applications (not FSAs) approved prior to the update. eIRB User Impact Q: When will eIRB2 go live? Monday, January 27, 2014 Migration of data from eIRB1 eIRB 2 will occur over a weekend to minimize user impact. What’s New in eIRB2? Major Changes: Modernized “Look and Feel” Application Numbers New Application Study Team Members Recruitment Drugs Devices Imaging/Radiation Updating & Deleting Documents Reviewer Notes Further Study Actions (FSAs) Protocol Event Reporting Application Numbers New Format Existing Applications will keep the NA_000xxxxx format New Application (Study Team) We’ve added a “User ID” filter to the “Select Person” window This will assist with adding a study team member who has the name first and last name as another eIRB user. New Application (Recruitment) We’ve moved “Recruitment Sources”, “Data Sources” and “HIPAA Form 4” to the Recruitment Information page. We’ve added additional questions help investigators determine: Can I recruit these individuals? Do I need a HIPAA Form 4? Choosing source(s) of recruitment will determine which questions appear. New Application (Recruitment) Example: New Application (Drugs) “Drug Information” & “Drug Storage” were merged. If “Yes” to Drugs, additional questions/sub-questions may be required: New Application (Devices) No More “Drill Downs” (pages within pages) New Application (Devices) If “Yes” to Devices, select from four (4) types of Devices: 1. FDA marketing clearance and is used for FDA-approved indication 2. Investigational Devices Exemption (IDE) 3. Non-Significant Risk (NSR) or Exempt from IDE Requirements 4. Humanitarian Device Exemption (HDE) for a Humanitarian Use Device (HUD) Complete the subsequent questions. All done! New Application (Imaging/Radiation) Radiation Worksheets (RCU-5) are now part of the eIRB application. (27) Radiation Exposure (28) Radionuclide Worksheet (29) External Radiation Exposure Worksheet Application Triggers 25 – Imaging/Radiation, Q5 and/or Q6 = “Yes”, and Category is “research” or “research and standard of care” **If ALL standard of care, no radiation calculator/worksheets are required** Updating & Deleting Documents Updating Documents “Upload Revision” is now “Update” You must select “Update” to: View the history of a document Delete a document Always Remember to… Use “Add” to upload a New Document Use “Update” to upload a Revised Document Updating & Deleting Documents Deleting Documents No more “Delete” button Use “Update” to change the status of the document to “Deleted” A “strikethrough” will appear through the document name. Use “Update” to upload the correct document Remember to change the status to “Submitted” before clicking “OK”. Reviewer Notes Reviewer Notes have replaced the “Respond to Issues” activity. IRB staff will add reviewer notes to the applicable section of the eIRB application. Investigators will be required to make revisions to the application (if applicable) and/or provide a response/explanation in the text box provided. A response is required for each note prior to submission. “Next” allows the investigator to move seamlessly from one note to the next. “Previous” allows the investigator to move back to a previous note. To edit your response, click on “Study Team Response”. Click “Ok” to save your response. Your response will be highlighted in green when complete. You can also track and respond to reviewer notes using the “Reviewer Notes” tab on the Application Workspace. Further Study Actions (FSAs) Choose the FSA type (you can only choose one at a time): ○ Change in Research ○ Continuing Review ○ Emergency Use Request ○ Protocol Event ○ Termination Report Click “Continue” to start the FSA submission. Change in Research no more “Click here to go to the application” link. Protocol Event Reporting Formerly “Problem Events” Only one event per submission – if multiple participants are involved in a specific event, include information for ALL participants. We’ve added five questions to assist the investigator in determining whether the event requires submission to the IRB. The protocol event may still be required to be submitted as a protocol deviation. Contact the IRB office if you have questions. Looking Forward… Current URL (http://e-irb.jhmi.edu) will remain the same – will point to eIRB2 URL for eIRB1 will change -- a link back to eIRB1 will be accessible from your investigator workspace. Questions? Concerns? eIRB Help Desk jhmeirb@jhmi.edu 410-502-2092 Janelle Maddox-Regis IRB Training Manager jmaddox3@jhmi.edu 410-502-0376